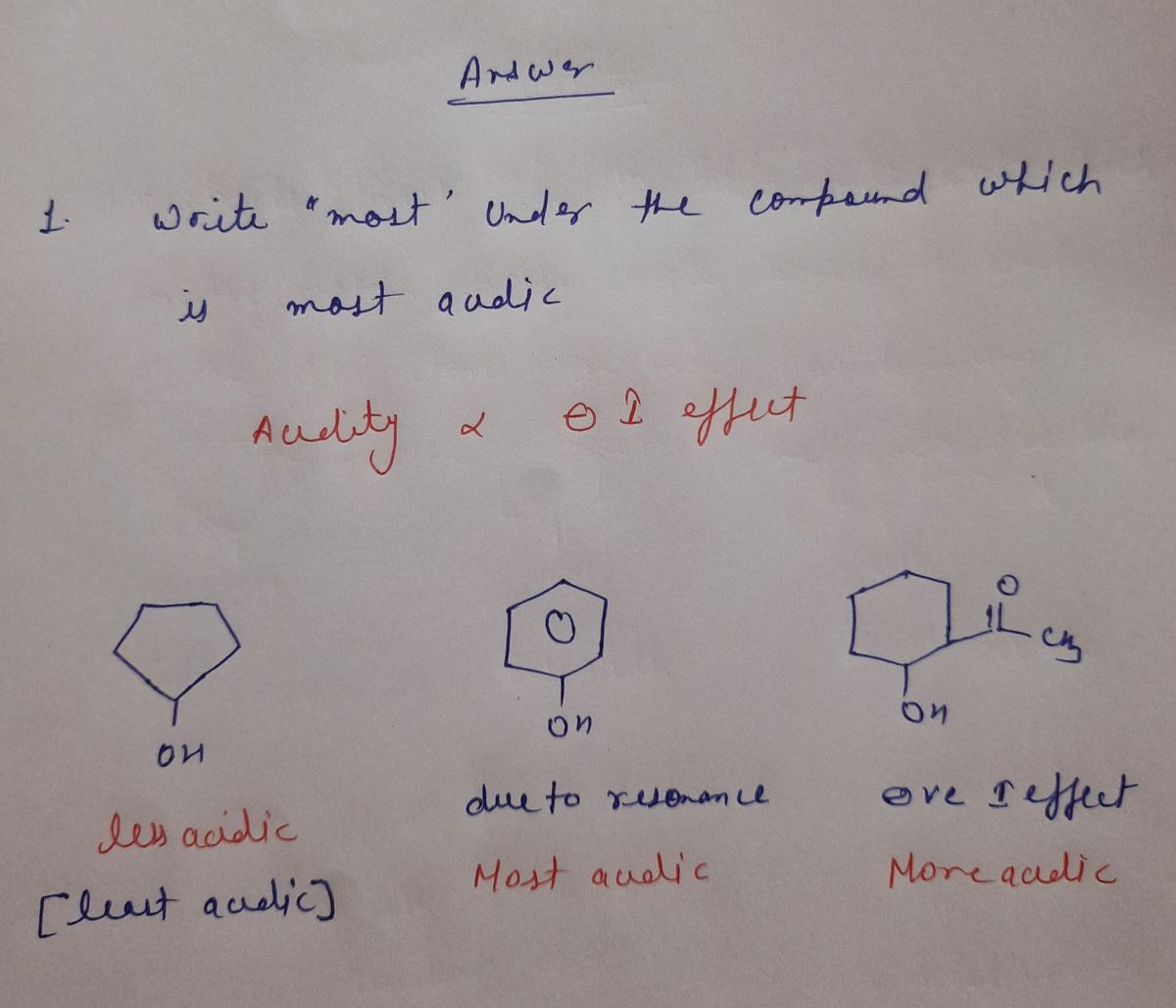
Chemistry 204 Homework Alcohols, Phenols, Ethers . Write “most" under the compound which is most acidic. Write "least" under the compound which is least acidic. ECH3 OH 11.00 in
Chemistry 204 Homework Alcohols, Phenols, Ethers . Write “most" under the compound which is most acidic. Write "least" under the compound which is least acidic. ECH3 OH 11.00 in
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Chemistry 204 Homework: Alcohols, Phenols, Ethers**
1. Write “most” under the compound which is most acidic. Write “least” under the compound which is least acidic.
**Compounds:**
- **Compound 1:** Cyclopentanol (a cyclopentane ring with an OH group attached).
- **Compound 2:** Phenol (a benzene ring with an OH group attached).
- **Compound 3:** 4-Methylcyclohexanone (a cyclohexane ring with an OH group and a ketone group (C=O) with a methyl group (CH₃) attached).
**Notes on Acidity:**
- Phenol is generally more acidic than alcohols like cyclopentanol due to resonance stabilization of the phenoxide ion.
- The compound with both an alcohol and a ketone may vary in acidity based on structure and substituents.
**Task:**
- Identify and label the most acidic and least acidic compounds based on the given structures.
![Transcription:
---
2. Write in the correct reagent needed to accomplish this reaction.
\[ \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \rightarrow \text{CH}_3\text{CH}_2\text{CH}_2\text{Cl} \]
3. Write in all the product(s) of this reaction. Label products as "most relevant."
\[ \text{CH}_3\text{CH}_2\text{CHCH}_3 \xrightarrow{\text{H}_2\text{SO}_4, \text{heat}} \]
4. Name this ether correctly.
---](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F60a763da-0d80-48cc-b9af-a1764b06c188%2F069b4d4e-0c48-42d3-90e4-81c8f878b97c%2Ftdbqjgn_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Transcription:
---
2. Write in the correct reagent needed to accomplish this reaction.
\[ \text{CH}_3\text{CH}_2\text{CH}_2\text{OH} \rightarrow \text{CH}_3\text{CH}_2\text{CH}_2\text{Cl} \]
3. Write in all the product(s) of this reaction. Label products as "most relevant."
\[ \text{CH}_3\text{CH}_2\text{CHCH}_3 \xrightarrow{\text{H}_2\text{SO}_4, \text{heat}} \]
4. Name this ether correctly.
---
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY