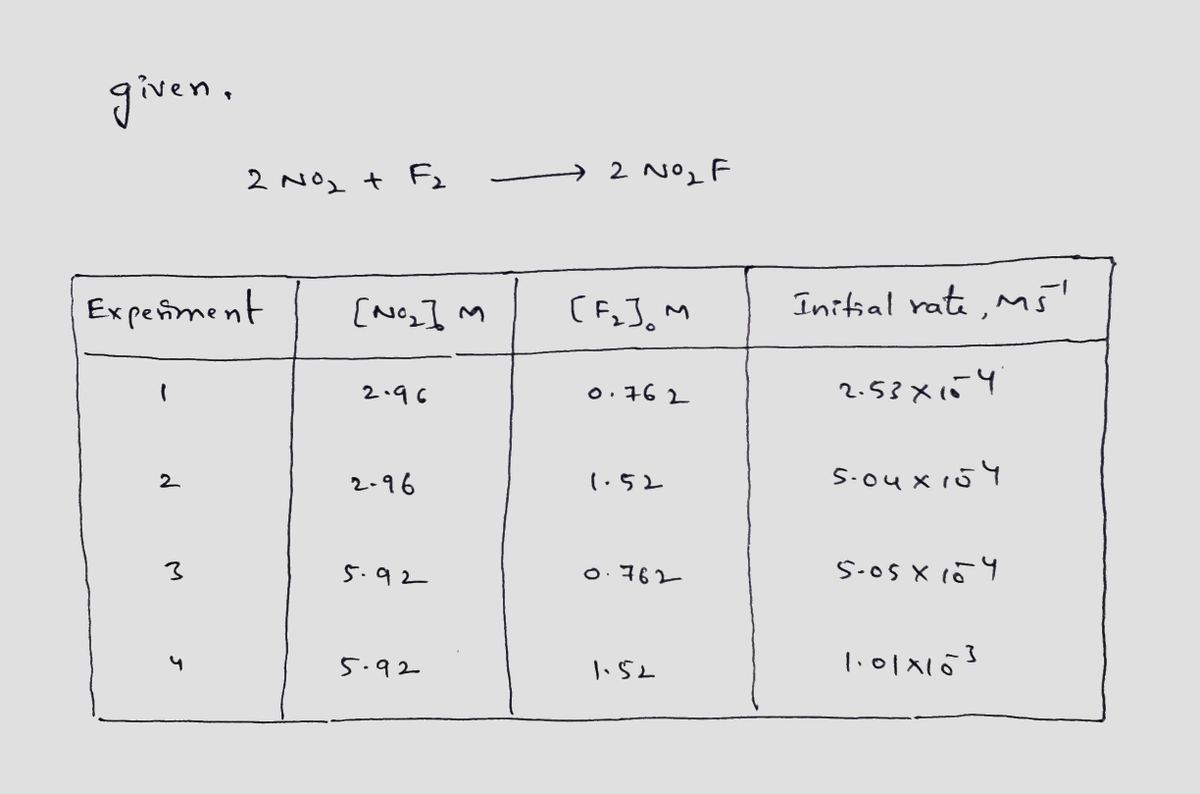
Car note CAU EMAIL CANVAS Pirate Ship MYJSCC BLACKBOARD Vacancies | A.| Birmingham Paraphrasing ..ewriting Tool Bb Content C OWLV2 | Online teaching and learning resour m Students Home [References] Use the References to access important values needed for this question. The following initial rate data are for the reaction of nitrogen dioxide with fluorine: 2 NO, + F, → 2 NO,F Experiment INO2lo, M [F],, M Initial Rate, Ms 1. 2.96 0.762 2.53x104 2.96 1.52 5.04x104 5.05x104 1.01x10-3 3 5.92 0.762 4 5.92 1.52 Complete the rate law for this reaction in the box below. Use the form k[A]"[B]", where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n Rate = From these data, the rate constant is M's!. Submit Answer Try Another Version 1 item attempt remaining
Car note CAU EMAIL CANVAS Pirate Ship MYJSCC BLACKBOARD Vacancies | A.| Birmingham Paraphrasing ..ewriting Tool Bb Content C OWLV2 | Online teaching and learning resour m Students Home [References] Use the References to access important values needed for this question. The following initial rate data are for the reaction of nitrogen dioxide with fluorine: 2 NO, + F, → 2 NO,F Experiment INO2lo, M [F],, M Initial Rate, Ms 1. 2.96 0.762 2.53x104 2.96 1.52 5.04x104 5.05x104 1.01x10-3 3 5.92 0.762 4 5.92 1.52 Complete the rate law for this reaction in the box below. Use the form k[A]"[B]", where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n Rate = From these data, the rate constant is M's!. Submit Answer Try Another Version 1 item attempt remaining
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![Car note
CAU EMAIL
CANVAS
Pirate Ship
MYJSCC
BLACKBOARD
Vacancies | A...| Birmingham
Paraphrasing ...ewriting Tool
Bb Content
C OWLV2 | Online teaching and learning resour
Students Home
[References]
Use the References to access important values if needed for this question.
The following initial rate data are for the reaction of nitrogen dioxide with fluorine:
2 NO2 + F2 → 2 NO,F
Experiment
[NO2]0, M
[F2]o, M
Initial Rate, Ms
1
2.96
0.762
2.53x10-4
2.96
1.52
5.04x10-4
3
5.92
0.762
5.05x10-4
4
5.92
1.52
1.01x103
Complete the rate law for this reaction in the box below.
Use the form k[A]"[B]" , where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n
Rate =
%3D
From these data, the rate constant is
Mls1.
Submit Answer
Try Another Version
1 item attempt remaining](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6d357038-3d1a-4d0e-a75d-dd3b105e8d20%2Fce2225aa-5ea4-4dfa-b3bc-d86a06ddc131%2Fbiy7wkg_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Car note
CAU EMAIL
CANVAS
Pirate Ship
MYJSCC
BLACKBOARD
Vacancies | A...| Birmingham
Paraphrasing ...ewriting Tool
Bb Content
C OWLV2 | Online teaching and learning resour
Students Home
[References]
Use the References to access important values if needed for this question.
The following initial rate data are for the reaction of nitrogen dioxide with fluorine:
2 NO2 + F2 → 2 NO,F
Experiment
[NO2]0, M
[F2]o, M
Initial Rate, Ms
1
2.96
0.762
2.53x10-4
2.96
1.52
5.04x10-4
3
5.92
0.762
5.05x10-4
4
5.92
1.52
1.01x103
Complete the rate law for this reaction in the box below.
Use the form k[A]"[B]" , where '1' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n
Rate =
%3D
From these data, the rate constant is
Mls1.
Submit Answer
Try Another Version
1 item attempt remaining
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY