Calculation of the equilibriun. Composition CO(g) + H₂0G) = (O₂(g) + th (g) -2 KOF) stemperature Yea Yt₂ усочно ист) where VI(T) is the reaction equilibrium Constant. At T²1105 K₁ KK ²1.00 Suppose the feed to a reactor contains. 1.00 mal of CO 2.00 mol of Hell, and no C0₂2 or the and the reaction mixture comer to equilibrium at 1105k. Calculate the equilibrium composition and the fractional conversion of the limiting. reactant.
Calculation of the equilibriun. Composition CO(g) + H₂0G) = (O₂(g) + th (g) -2 KOF) stemperature Yea Yt₂ усочно ист) where VI(T) is the reaction equilibrium Constant. At T²1105 K₁ KK ²1.00 Suppose the feed to a reactor contains. 1.00 mal of CO 2.00 mol of Hell, and no C0₂2 or the and the reaction mixture comer to equilibrium at 1105k. Calculate the equilibrium composition and the fractional conversion of the limiting. reactant.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
chemical processes. This problem has a quadratic expression. Be thorough to the fullest with the calculations.
![**Title: Calculation of the Equilibrium Composition**
**Chemical Reaction:**
\[ \text{CO(g) + 2H}_2\text{O(g)} \rightleftharpoons \text{CO}_2\text{(g) + H}_2\text{(g)} \]
**Equilibrium Constant Expression:**
\[ \text{K} = \frac{y_{\text{CO}_2} \times y_{\text{H}_2}}{y_{\text{CO}} \times (y_{\text{H}_2\text{O}})^2} \]
**Task:**
Suppose the feed for a reactor contains 25.0 mol of CO and 20.0 mol of H₂O, and no CO₂ or H₂. The reaction is to take place at equilibrium at 1105 K.
**Equilibrium Calculation:**
Given \[ \text{K} = 2.106 \] at 1105 K, calculate the equilibrium composition and the fractional conversion of the limiting reactant.
---
This note provides guidance on calculating the equilibrium composition for a given chemical reaction, using given initial quantities and the equilibrium constant at a specific temperature (1105 K).](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9ba88ecd-654b-4f6b-9783-e93c33ca6a16%2Faefabb87-18c6-4962-902b-2c78fe371052%2F4281rom_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Title: Calculation of the Equilibrium Composition**
**Chemical Reaction:**
\[ \text{CO(g) + 2H}_2\text{O(g)} \rightleftharpoons \text{CO}_2\text{(g) + H}_2\text{(g)} \]
**Equilibrium Constant Expression:**
\[ \text{K} = \frac{y_{\text{CO}_2} \times y_{\text{H}_2}}{y_{\text{CO}} \times (y_{\text{H}_2\text{O}})^2} \]
**Task:**
Suppose the feed for a reactor contains 25.0 mol of CO and 20.0 mol of H₂O, and no CO₂ or H₂. The reaction is to take place at equilibrium at 1105 K.
**Equilibrium Calculation:**
Given \[ \text{K} = 2.106 \] at 1105 K, calculate the equilibrium composition and the fractional conversion of the limiting reactant.
---
This note provides guidance on calculating the equilibrium composition for a given chemical reaction, using given initial quantities and the equilibrium constant at a specific temperature (1105 K).
Expert Solution
Step 1: Given data
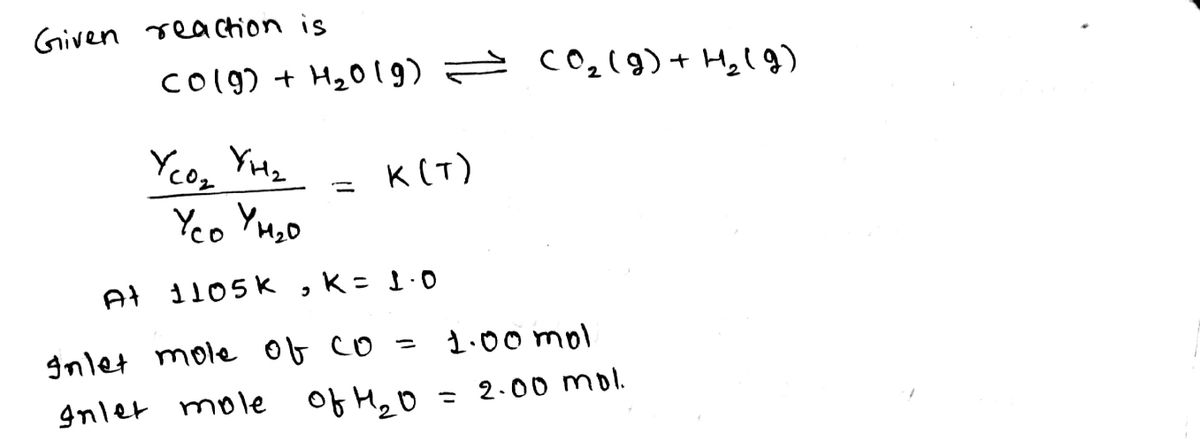
Step by step
Solved in 4 steps with 5 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The