Calculate the pH of a buffer that is 0.020 M in NH3 and 0.030M in NH4Cl What is the pH after adding 1.00 mL of 0.01 M NaOH to 0.10 L of this buffer? [Ka for NH+4 if 5.70 x 10^-10]
Calculate the pH of a buffer that is 0.020 M in NH3 and 0.030M in NH4Cl What is the pH after adding 1.00 mL of 0.01 M NaOH to 0.10 L of this buffer?
[Ka for NH+4 if 5.70 x 10^-10]
Buffers contain the ability for neutralizing the added acids/alkali's amounts in the solution and also maintain that solution's pH stable. Acidic buffers mainly tend to neutralize basic solution whereas basic buffers tend to neutralize acidic solution.
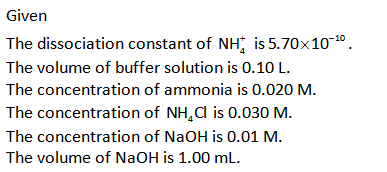
The Henderson Hasselbalch equation for the basic buffer is shown below.
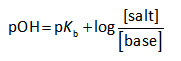
The reaction for the formation of the buffer is shown below.
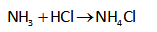
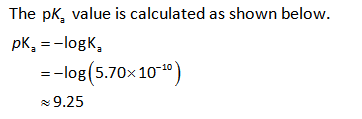
The Henderson Hasselbalch equation for the calculation of the pH of basic buffer can be written as,
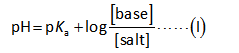
Substitute all the known values in the formula.
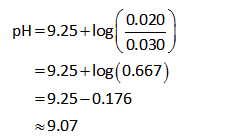
Therefore, the pH of the buffer solution is 9.07.
Calculation of pH after the addition of 1.00 mL of 0.01 M NaOH:
The NaOH will react with ammonium ion furnished by the salt and produces ammonia.
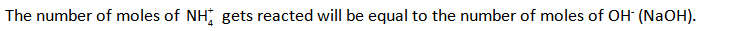
The number of moles of NaOH can be calculated as shown below.
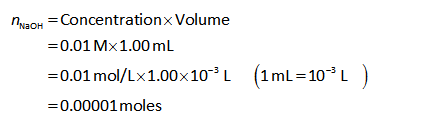

Step by step
Solved in 7 steps with 14 images









