Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Calculate the number of photons of light with a wavelength of 3000 pm that provide 2 J of energy.
Expert Solution
Step 1
It is required to calculate the total number of photons having a total energy of 2 J. Energy of one photon can be calculated as,
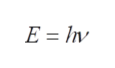
Step 2
Here, ‘h’ is Planck’s constant and ‘ν’ is the frequency of radiation. Expressing ν in terms of speed of light (c) and wavelength (λ),
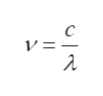
Step 3
Substituting the value of ‘ν’ in equation, we get the energy of one photon, which is given as,
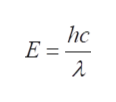
Step 4
The equation obtained in the previous step is the energy of a single photons and for ‘N’ photons, the total energy is given as,
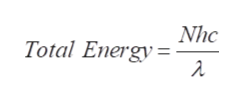
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
Expert Answers to Latest Homework Questions
Q: Using the chart describe the change from cystine to tyrosine and its impact on the protein.
Using…
Q: Please help me fill out the chart then using the chart describe the change from cystine to tyrosine…
Q: Write the Esterification reaction mechanism for acetic acid, and one propanol to make propanol…
Q: Which of these compounds is Ester formed from the reaction of acetic acid and one propanol 
Q: Describe the four labeled parts of the reaction diagram from the reaction of sucrose, breaking down…
Q: 4.
Use this information to answer Question 4-8:
Consider a step pn junction made of GaAs at T = 300…
Q: 1.
Use this information to answer Question 1-3:
Consider a step pn junction made of silicon. The p-…
Q: microprocers and microcontroler
Q: What is EBITDA, and why is it often used as a proxy for cash flow?
Q: Please provide the correct answer to this general accounting problem using valid calculations.
Q: What is the difference between alpha and beta in investment analysis? Need help
Q: Can you help me solve this general accounting problem using the correct accounting process?
Q: What is a derivative, and how is it used for hedging? Need help
Q: Explain the Capital Asset Pricing Model (CAPM). What does each component represent in Finance ?
Q: What is the difference between alpha and beta in investment analysis?
No AI
Q: What is a derivative, and how is it used for hedging in finance?
Q: What is the difference between alpha and beta in investment analysis?
Q: Hello tutor solve this question and accounting question
Q: I want the correct answer and accounting question
Q: Provide correct solution and accounting question
Q: I want the correct answer with general accounting question