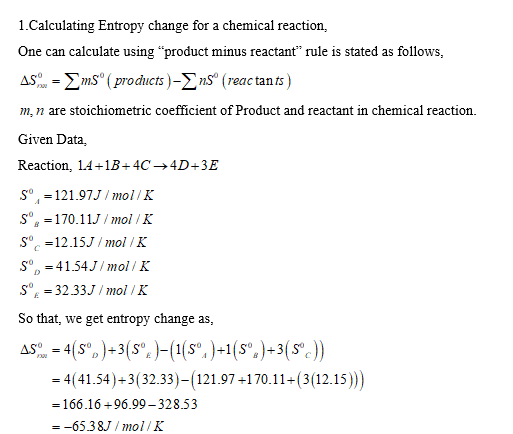
Calculate the Gibbs free energy (in kJ) at 25 degrees C of the following reaction using the data in the table. 1A +1B + 4C - 4D + 3E AHF°(kJ/mol) sOU/mol/K) A -82.84 121.97 B 91.68 C -89.37 170.11 12.15 D -88.02 41.54 E 64.03 32.33
Calculate the Gibbs free energy (in kJ) at 25 degrees C of the following reaction using the data in the table. 1A +1B + 4C - 4D + 3E AHF°(kJ/mol) sOU/mol/K) A -82.84 121.97 B 91.68 C -89.37 170.11 12.15 D -88.02 41.54 E 64.03 32.33
Introductory Circuit Analysis (13th Edition)
13th Edition
ISBN:9780133923605
Author:Robert L. Boylestad
Publisher:Robert L. Boylestad
Chapter1: Introduction
Section: Chapter Questions
Problem 1P: Visit your local library (at school or home) and describe the extent to which it provides literature...
Related questions
Concept explainers
KVL and KCL
KVL stands for Kirchhoff voltage law. KVL states that the total voltage drops around the loop in any closed electric circuit is equal to the sum of total voltage drop in the same closed loop.
Sign Convention
Science and technology incorporate some ideas and techniques of their own to understand a system skilfully and easily. These techniques are called conventions. For example: Sign conventions of mirrors are used to understand the phenomenon of reflection and refraction in an easier way.
Question
11.
![**Gibbs Free Energy Calculation for a Chemical Reaction**
**Objective:**
Calculate the Gibbs free energy (in kJ) at 25 degrees Celsius for the following chemical reaction using the data provided in the table.
**Chemical Reaction:**
\[ 1A + 1B + 4C \rightarrow 4D + 3E \]
**Data Table:**
| Species | \(\Delta H_f^0\) (kJ/mol) | \(S^0\) (J/mol·K) |
|---------|----------------|--------------|
| A | -82.84 | 121.97 |
| B | 91.68 | 170.11 |
| C | -89.37 | 12.15 |
| D | -88.02 | 41.54 |
| E | 64.03 | 32.33 |
**Explanation:**
- \(\Delta H_f^0\) represents the standard enthalpy of formation for each species.
- \(S^0\) denotes the standard entropy for each species.
- The given values are used to calculate the Gibbs free energy change (\(\Delta G\)) using the equation:
\[
\Delta G = \Delta H - T\Delta S
\]
Where:
- \(\Delta H = \Sigma (\Delta H_f^0 \, \text{products}) - \Sigma (\Delta H_f^0 \, \text{reactants})\)
- \(\Delta S = \Sigma (S^0 \, \text{products}) - \Sigma (S^0 \, \text{reactants})\)
- \(T\) is the temperature in Kelvin (25°C = 298 K).
**Instructions:**
1. Calculate \(\Delta H\) using the given enthalpies of formation.
2. Calculate \(\Delta S\) using the given entropies.
3. Use the Gibbs free energy equation to find \(\Delta G\).
This calculation will indicate the spontaneity of the reaction under standard conditions.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7a00ba11-4727-4380-bbb0-88ea42161338%2Fd32ebb52-1b83-4d27-bd05-aaa79ebda0af%2Fe7ado7.jpeg&w=3840&q=75)
Transcribed Image Text:**Gibbs Free Energy Calculation for a Chemical Reaction**
**Objective:**
Calculate the Gibbs free energy (in kJ) at 25 degrees Celsius for the following chemical reaction using the data provided in the table.
**Chemical Reaction:**
\[ 1A + 1B + 4C \rightarrow 4D + 3E \]
**Data Table:**
| Species | \(\Delta H_f^0\) (kJ/mol) | \(S^0\) (J/mol·K) |
|---------|----------------|--------------|
| A | -82.84 | 121.97 |
| B | 91.68 | 170.11 |
| C | -89.37 | 12.15 |
| D | -88.02 | 41.54 |
| E | 64.03 | 32.33 |
**Explanation:**
- \(\Delta H_f^0\) represents the standard enthalpy of formation for each species.
- \(S^0\) denotes the standard entropy for each species.
- The given values are used to calculate the Gibbs free energy change (\(\Delta G\)) using the equation:
\[
\Delta G = \Delta H - T\Delta S
\]
Where:
- \(\Delta H = \Sigma (\Delta H_f^0 \, \text{products}) - \Sigma (\Delta H_f^0 \, \text{reactants})\)
- \(\Delta S = \Sigma (S^0 \, \text{products}) - \Sigma (S^0 \, \text{reactants})\)
- \(T\) is the temperature in Kelvin (25°C = 298 K).
**Instructions:**
1. Calculate \(\Delta H\) using the given enthalpies of formation.
2. Calculate \(\Delta S\) using the given entropies.
3. Use the Gibbs free energy equation to find \(\Delta G\).
This calculation will indicate the spontaneity of the reaction under standard conditions.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, electrical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Circuit Analysis (13th Edition)
Electrical Engineering
ISBN:
9780133923605
Author:
Robert L. Boylestad
Publisher:
PEARSON

Delmar's Standard Textbook Of Electricity
Electrical Engineering
ISBN:
9781337900348
Author:
Stephen L. Herman
Publisher:
Cengage Learning

Programmable Logic Controllers
Electrical Engineering
ISBN:
9780073373843
Author:
Frank D. Petruzella
Publisher:
McGraw-Hill Education

Introductory Circuit Analysis (13th Edition)
Electrical Engineering
ISBN:
9780133923605
Author:
Robert L. Boylestad
Publisher:
PEARSON

Delmar's Standard Textbook Of Electricity
Electrical Engineering
ISBN:
9781337900348
Author:
Stephen L. Herman
Publisher:
Cengage Learning

Programmable Logic Controllers
Electrical Engineering
ISBN:
9780073373843
Author:
Frank D. Petruzella
Publisher:
McGraw-Hill Education

Fundamentals of Electric Circuits
Electrical Engineering
ISBN:
9780078028229
Author:
Charles K Alexander, Matthew Sadiku
Publisher:
McGraw-Hill Education

Electric Circuits. (11th Edition)
Electrical Engineering
ISBN:
9780134746968
Author:
James W. Nilsson, Susan Riedel
Publisher:
PEARSON

Engineering Electromagnetics
Electrical Engineering
ISBN:
9780078028151
Author:
Hayt, William H. (william Hart), Jr, BUCK, John A.
Publisher:
Mcgraw-hill Education,