Calculate the energy required to heat 1.00 kg of graphite from 1.0 °C to 23.3 °C. Assume the specific heat capacity of - 1 graphite under these conditions is 0.710 J-g K'. Round your answer to 3 significant digits.
Calculate the energy required to heat 1.00 kg of graphite from 1.0 °C to 23.3 °C. Assume the specific heat capacity of - 1 graphite under these conditions is 0.710 J-g K'. Round your answer to 3 significant digits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
calculate the energy required to heat graphite with the information from the picture
![**Energy Calculation for Heating Graphite**
**Problem Statement:**
Calculate the energy required to heat 1.00 kg of graphite from 1.0 °C to 23.3 °C. Assume the specific heat capacity of graphite under these conditions is 0.710 J·g⁻¹·K⁻¹. Round your answer to 3 significant digits.
**Variables:**
- **Mass (m):** 1.00 kg (1000 g)
- **Initial Temperature (T₁):** 1.0 °C
- **Final Temperature (T₂):** 23.3 °C
- **Specific Heat Capacity (c):** 0.710 J·g⁻¹·K⁻¹
**Approach:**
To find the energy, use the formula:
\[ Q = m \cdot c \cdot \Delta T \]
Where:
- \( Q \) is the heat energy required,
- \( \Delta T = T₂ - T₁ \) is the change in temperature.
**Solution:**
1. Calculate the change in temperature:
\[ \Delta T = 23.3 °C - 1.0 °C = 22.3 °C \]
2. Substitute the values into the formula:
\[ Q = 1000 \, \text{g} \times 0.710 \, \text{J·g⁻¹·K⁻¹} \times 22.3 \, \text{K} \]
3. Compute the result and round to 3 significant digits.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F1e77a53e-015e-4d74-8706-0af5e82649c9%2F0f8b3857-5706-4897-a9cd-4096a80e845d%2Ftrwe1sk_processed.png&w=3840&q=75)
Transcribed Image Text:**Energy Calculation for Heating Graphite**
**Problem Statement:**
Calculate the energy required to heat 1.00 kg of graphite from 1.0 °C to 23.3 °C. Assume the specific heat capacity of graphite under these conditions is 0.710 J·g⁻¹·K⁻¹. Round your answer to 3 significant digits.
**Variables:**
- **Mass (m):** 1.00 kg (1000 g)
- **Initial Temperature (T₁):** 1.0 °C
- **Final Temperature (T₂):** 23.3 °C
- **Specific Heat Capacity (c):** 0.710 J·g⁻¹·K⁻¹
**Approach:**
To find the energy, use the formula:
\[ Q = m \cdot c \cdot \Delta T \]
Where:
- \( Q \) is the heat energy required,
- \( \Delta T = T₂ - T₁ \) is the change in temperature.
**Solution:**
1. Calculate the change in temperature:
\[ \Delta T = 23.3 °C - 1.0 °C = 22.3 °C \]
2. Substitute the values into the formula:
\[ Q = 1000 \, \text{g} \times 0.710 \, \text{J·g⁻¹·K⁻¹} \times 22.3 \, \text{K} \]
3. Compute the result and round to 3 significant digits.
Expert Solution
Step 1
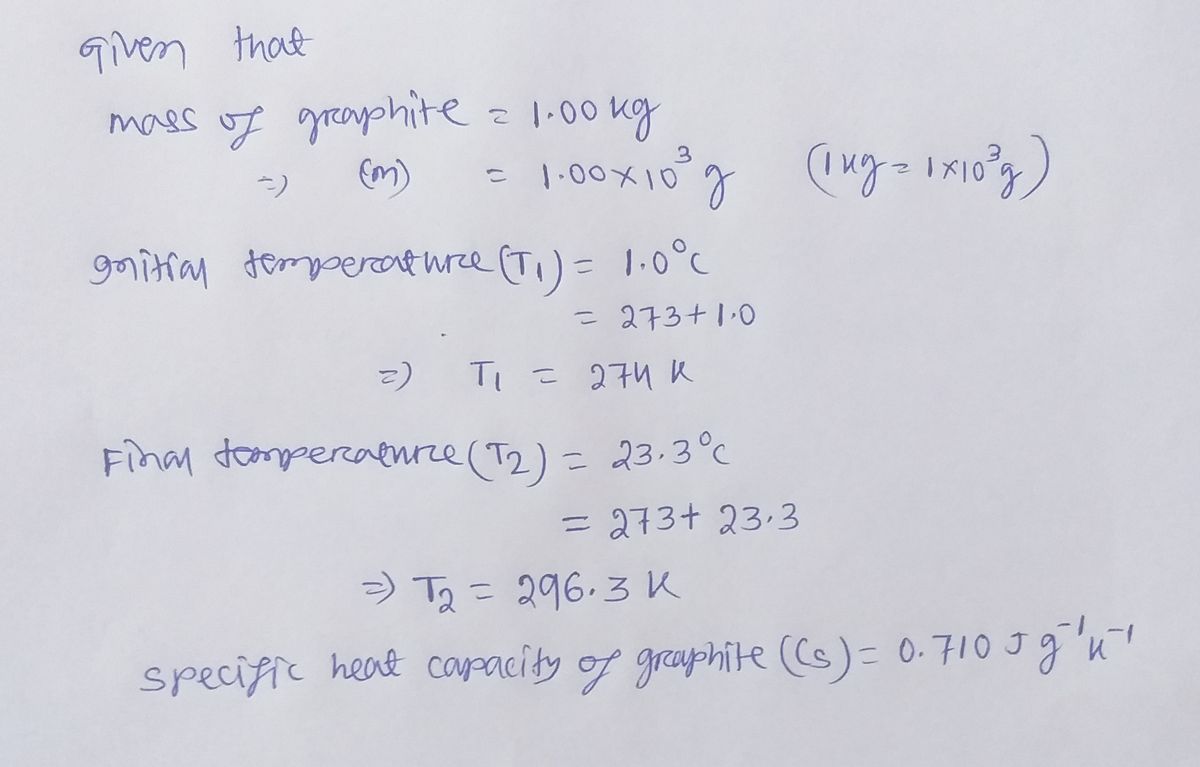
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY