Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Please help me this problem! Thank you!
Please show the mechanics of the reactions below

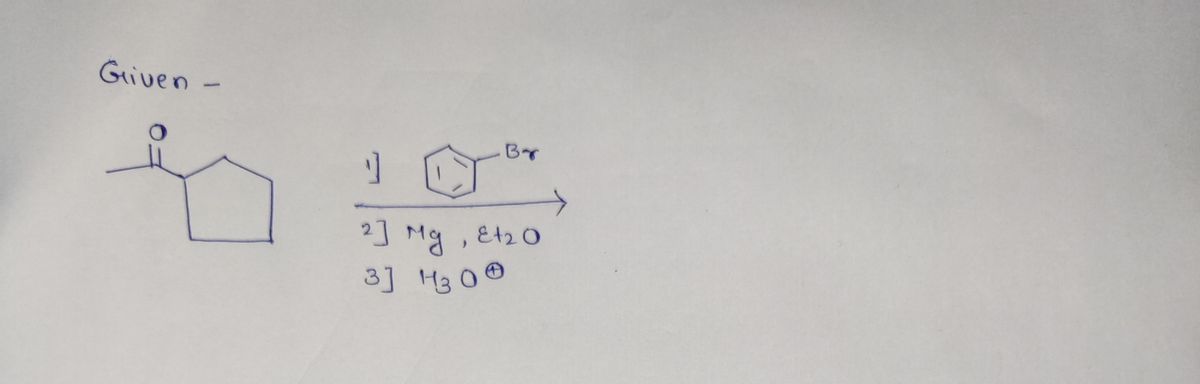
Transcribed Image Text:### Chemical Reaction Scheme
This image depicts a multi-step organic reaction process. Below is a transcription of the reaction scheme with detailed explanations:
#### Reactants and Reagents:
1. **Starting Material:**
- Cyclopentanone
- It consists of a five-membered carbon ring with a ketone group (C=O) attached.
2. **Reagents:**
- **Step 1:** A bromobenzene derivative.
- The benzene ring is substituted with a bromine atom (Br).
- **Step 2:** Magnesium (Mg) in the presence of diethyl ether (Et₂O).
- This step likely involves the formation of a Grignard reagent.
- **Step 3:** Hydronium ion (H₃O⁺).
- This is typically used for an aqueous acidic workup.
#### Reaction Process:
1. **Formation of the Grignard Reagent:**
- The bromobenzene derivative reacts with magnesium in diethyl ether to form a Grignard reagent. Grignard reagents are organomagnesium compounds crucial for further synthetic reactions.
2. **Reaction with Cyclopentanone:**
- The Grignard reagent generated in the previous step reacts with cyclopentanone. This reaction typically leads to the formation of a tertiary alcohol upon the addition of the Grignard reagent to the carbonyl group.
3. **Acidic Workup:**
- The final step involves the addition of an acidic solution (H₃O⁺) to protonate the oxygen and stabilize the alcohol product.
### Diagram Analysis:
- The image displays a sequence of chemical transformations, typically presented with arrows indicating progression, alongside chemical structures briefly annotated with reagents and conditions.
This reaction is representative of common synthetic strategies within organic chemistry, showcasing the utility of Grignard reagents in constructing complex molecular architectures.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY