Boyle's Law Ľ states that for a certain gas in a container we have P. V = 345 where P represents the pressure of the gas (in mmHG) and V represents the volume of the gas (in liters). a. If the pressure of the gas is 220 mmHG, what is the volume of the gas? liters Preview b. Write a function f that determines the volume of the gas (in liters) in terms of the pressure of the gas in mmHG, P. f(P) = Preview c. Complete the following statement. (Hint: if f(P) increases without bound, enter "oo". If f(P) decreases without bound, enter "- o0".) As P → 0*, f(P) → Preview
Boyle's Law Ľ states that for a certain gas in a container we have P. V = 345 where P represents the pressure of the gas (in mmHG) and V represents the volume of the gas (in liters). a. If the pressure of the gas is 220 mmHG, what is the volume of the gas? liters Preview b. Write a function f that determines the volume of the gas (in liters) in terms of the pressure of the gas in mmHG, P. f(P) = Preview c. Complete the following statement. (Hint: if f(P) increases without bound, enter "oo". If f(P) decreases without bound, enter "- o0".) As P → 0*, f(P) → Preview
Calculus: Early Transcendentals
8th Edition
ISBN:9781285741550
Author:James Stewart
Publisher:James Stewart
Chapter1: Functions And Models
Section: Chapter Questions
Problem 1RCC: (a) What is a function? What are its domain and range? (b) What is the graph of a function? (c) How...
Related questions
Question

Transcribed Image Text:Boyle's Law states that for a certain gas in a
container we have P· V = 345 where P
represents the pressure of the gas (in mmHG)
and V represents the volume of the gas (in liters).
a. If the pressure of the gas is 220 mmHG,
what is the volume of the gas?
liters
Preview
b. Write a function f that determines the
volume of the gas (in liters) in terms of the
pressure of the gas in mmHG, P.
f(P) =
Preview
c. Complete the following statement. (Hint: if
f(P) increases without bound, enter "oo".
If f(P) decreases without bound, enter "-
o0".)
As P → 0*, f(P) →
Preview
Expert Solution
Step 1
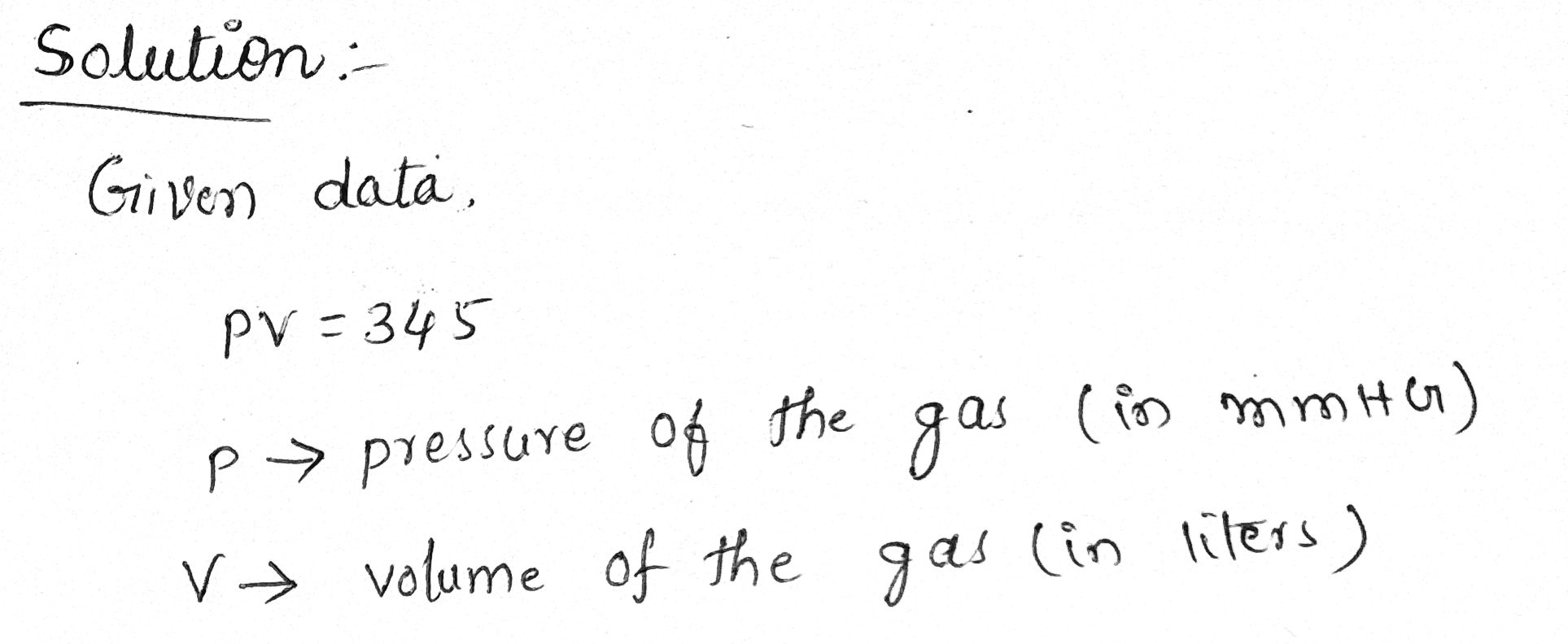
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781285741550
Author:
James Stewart
Publisher:
Cengage Learning

Thomas' Calculus (14th Edition)
Calculus
ISBN:
9780134438986
Author:
Joel R. Hass, Christopher E. Heil, Maurice D. Weir
Publisher:
PEARSON

Calculus: Early Transcendentals (3rd Edition)
Calculus
ISBN:
9780134763644
Author:
William L. Briggs, Lyle Cochran, Bernard Gillett, Eric Schulz
Publisher:
PEARSON

Calculus: Early Transcendentals
Calculus
ISBN:
9781319050740
Author:
Jon Rogawski, Colin Adams, Robert Franzosa
Publisher:
W. H. Freeman


Calculus: Early Transcendental Functions
Calculus
ISBN:
9781337552516
Author:
Ron Larson, Bruce H. Edwards
Publisher:
Cengage Learning