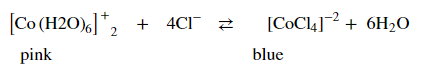Chemistry by OpenStax (2015-05-04) 1st Edition
ISBN: 9781938168390
Author: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher: Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
1 Essential Ideas 2 Atoms, Molecules, And Ions 3 Composition Of Substances And Solutions 4 Stoichiometry Of Chemical Reactions 5 Thermochemistry 6 Electronic Structure And Periodic Properties Of Elements 7 Chemical Bonding And Molecular Geometry 8 Advanced Theories Of Covalent Bonding 9 Gases 10 Liquids And Solids 11 Solutions And Colloids 12 Kinetics 13 Fundamental Equilibrium Concepts 14 Acid-base Equilibria 15 Equilibria Of Other Reaction Classes 16 Thermodynamics 17 Electrochemistry 18 Representative Metals, Metalloids, And Nonmetals 19 Transition Metals And Coordination Chemistry 20 Organic Chemistry 21 Nuclear Chemistry Chapter15: Equilibria Of Other Reaction Classes
Chapter Questions Section: Chapter Questions
Problem 1E: Complete the changes in concentrations for each of the following reactions: (a) AgI(s)Ag+(aq)+I(aq)x... Problem 2E: Complete the changes in concentrations for each of the following reactions: (a)... Problem 3E: How do the concentrations of Ag+ and CrO42- in a saturated solution above 1.0 g of solid Ag2CrO4... Problem 4E: How do the concentrations of Pb2+ and S2- change when K2S is added to a saturated solution of PbS? Problem 5E: What additional information do we need to answer the following question: How is the equilibrium of... Problem 6E: Which of the following slightly soluble compounds has a solubility greater than that calculated from... Problem 7E: Which of the following slightly soluble compounds has a solubility greater than that calculated from... Problem 8E: Write the ionic equation for dissolution and the solubility product (Ksp) expression for each of the... Problem 9E: Write the ionic equation for the dissolution and the Ksp expression for each of the following... Problem 10E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities... Problem 11E: The Handbook of Chemistry and Physics (http://openstaxcollege.org/l/16Handbook) gives solubilities... Problem 12E: Use solubility products and predict which of the following salts is the most soluble, in terms of... Problem 13E: Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of... Problem 14E: Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of... Problem 15E: Assuming that no equilibria other than dissolution are involved, calculate the concentration of all... Problem 16E: Assuming that no equilibria other than dissolution are involved, calculate the concentration of all... Problem 17E: Assuming that no equilibria other than dissolution are involved, calculate the concentration of all... Problem 18E: Explain why the changes in concentrations of the common ions in Exercise 15.17 can be neglected. Problem 19E: Explain why the Changes in concentrations of the common ions in Exercise 15.18 cannot be neglected. Problem 20E: Calculate the solubility of aluminum hydroxide, Al(OH)3, in a solution buffered at pH 11.00. Problem 21E: Refer to Appendix J for solubility products for calcium salts. Determine which of the calcium salts... Problem 22E: Most barium compounds are very poisonous; however, barium sulfate is often administered internally... Problem 23E: Public Health Service standards for drinking water set a maximum of 250 mg/L (2.620103M) of SO42-... Problem 24E: Perform the following calculations: (a) Calculate [Ag+] in a saturated aqueous solution of AgBr. (b)... Problem 25E: The solubility product of CaSO42H2O is 2.4105. What mass of this salt will dissolve in 1.0 L of... Problem 26E: Assuming that no equilibria other than dissolution are involved, calculate the concentrations of... Problem 27E: Assuming that no equilibria other than dissolution are involved, calculate the concentrations of... Problem 28E: The following concentrations are found in mixtures of ions in equilibrium with slightly soluble... Problem 29E: The following concentrations are found in mixtures of ions in equilibrium with slightly soluble... Problem 30E: Which of the following compounds precipitates from a solution that has the concentrations indicated?... Problem 31E: Which of the following compounds precipitates from a solution that has the concentrations indicated?... Problem 32E: Calculate the concentration of Tl+ when TICl just begins to precipitate from a solution that is... Problem 33E: Calculate the concentration of sulfate ion when BaSO4 just begins to precipitate from a solution... Problem 34E: Calculate the concentration of Sr2+ when SrF2 starts to precipitate from a solution that is 0.0025 M... Problem 35E: Calculate the concentration of PO43- when Ag3PO4 starts to precipitate from a solution that is... Problem 36E: Calculate the concentration of F- required to begin precipitation of CaF2 in a solution that is... Problem 37E: Calculate the concentration] of Ag+ required to begin precipitation of Ag2CO3 in a solution that is... Problem 38E: What [Ag+] is required to reduce [CO32-] to 8.2104M by precipitation of Ag2CO3? Problem 39E: What [F-] is required to reduce [Ca2+] to 1.0104M by precipitation of CaF2? Problem 40E: A volume of 0.800 L of a 2104 -M Ba(NO3)2 solution is added to 0.200 L of 5104M Li2SO4. Does BaSO4... Problem 41E: Perform these calculations for nickel(II) carbonate. (a) With what volume of water must a... Problem 42E: Iron concentrations greater than 5.4106 M in water used for laundry purposes can cause staining.... Problem 43E: A solution is 0.010 M in both Cu2+ and Cd2+. What percentage of Cd2+ remains in the solution when... Problem 44E: A solution is 0.15 M in both Pb2+ and Ag+. If Cl- is added to this solution, what is [Ag+] when... Problem 45E: What reagent might be used to separate the ions in each of the following mixtures, which are 0.1 M... Problem 46E: A solution contains 1.0105 mol of KBr and 0.10 mol of KCl per liter. AgNO3 is gradually added to... Problem 47E: A solution contains 1.0102 mol of Kl and 0.10 mol of KCl per liter. AgNO3 is gradually added to this... Problem 48E: The calcium ions in human blood serum are necessary for coagulation (Figure 15.5). Potassium... Problem 49E: About 50% of urinary calculi (kidney stones) consist of calcium phosphate, Ca3(PO4)2. The normal... Problem 50E: The pH of normal urine is 6.30, and the total phosphate concentration... Problem 51E: Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production... Problem 52E: Hydrogen sulfide is bubbled into a solution that is 0.10 M in both Pb2+ and Fe2+ and 0.30 M in HCl.... Problem 53E: Perform the following calculations involving concentrations of iodate ions: (a) The iodate ion... Problem 54E: Calculate the molar solubility of AgBr in 0.035 M NaBr (KSP=51013). Problem 55E: How many grams of Pb(OH)2 will dissolve in 500 mL of a 0.050-M PbCl2 solution (KSP=1.21015)? Problem 56E: Use the simulation (http://openstaxcollege.org/l/16solublesalts) from the earlier Link to Learning... Problem 57E: How many grams of Milk of Magnesia, Mg(OH)2 (s) (58.3 g/mol), would be soluble in 200 mL of water.... Problem 58E: Two hypothetical salts, LM2 and LQ, have the same molar solubility in H2O. If Ksp for LM2 is... Problem 59E: Which of the following carbonates will form first? Which of the following will form last? Explain.... Problem 60E: How many grams of Zn(CN)2(s) (117.44 g/mol) would be soluble in 100 mL of H2O? Include the balanced... Problem 61E: Under what circumstances, if any, does a sample of solid AgCl completely dissolve in pure water? Problem 62E: Explain why the addition of NH3 or HNO3 to a saturated solution of Ag2CO3 in contact with solid... Problem 63E: Calculate the cadmium ion concentration, [Cd2+], in a solution prepared by mixing 0.100 L of 0.0100... Problem 64E: Explain why addition of NH3 or HNO3 to a saturated solution of Cu(OH)2 in contact with solid Cu(OH)2... Problem 65E: Sometimes equilibria fur complex ions are described in terms of dissociation constants. Kd . For the... Problem 66E: Using the value of the formation constant for the complex ion Co(NH3)62+ calculate the dissociation... Problem 67E: Using the dissociation constant, Kd=7.81018, calculate the equilibrium concentrations of Cd2+ and... Problem 68E: Using the dissociation constant, Kd=3.41015, calculate the equilibrium concentrations of Zn2+ and... Problem 69E: Using the dissociation constant, Kd=2.21034, calculate the equilibrium concentrations of Co3+ and... Problem 70E: Using the dissociation constant kd=1*10-44 calculate the equilibrium concentrations of Fe3+ and CN-... Problem 71E: Calculate the mass of potassium cyanide ion that must be added to 100 mL of solution to dissolve... Problem 72E: Calculate the minimum concentration of ammonia needed in 1.0 L of solution to dissolve 3.0103 mol of... Problem 73E: A roll of 35-mm black and white photographic film contains about 0.27 g of unexposed AgBr before... Problem 74E: We have seen an introductory definition of an acid: An acid is a compound that reacts with water and... Problem 75E: Write the Lewis structures of the reactants and product of each of the following equations, and... Problem 76E: Write the Lewis structures of the reactants and product of each of the following equations, and... Problem 77E: Using Lewis structures, write balanced equations for the following reactions: (a) HCI(g)+PH3(g) (b)... Problem 78E: Calculate [HgCl42-] in a solution prepared by adding 0.0200 mol of NaCl to 0.250 L of a 0.100-M... Problem 79E: In a titration of cyanide ion, 28.72 mL of 0.0100 M AgNO3 is added before precipitation begins. [The... Problem 80E: What are the concentrations of Ag+, CN-, and Ag(CN)2- in a saturated solution of AgCN? Problem 81E: In dilute aqueous solution HF acts as a weak acid. However, pure liquid HF (boiling point = 19.5 C)... Problem 82E: The simplest amino acid is glycine, H2NCH2CO2H. The common feature of amino acids is that they... Problem 83E: Boric acid, H3303, is not a Bronsted-Lowry acid but a Lewis acid.. (a) Write an equation for its... Problem 84E: A saturated solution of a slightly soluble electrolyte in contact with some of the solid electrolyte... Problem 85E: Calculate the equilibrium concentration of Ni2+ in a 1.0-M solution [Ni(NH3)6](NO3)2. Problem 86E: Calculate the equilibrium concentration of Zn2+ in a 0.30-M solution of Zn(CN)42-. Problem 87E: Calculate the equilibrium concentration of Cu2+ in a solution initially with 0.050 M Cu2+ and 1.00 M... Problem 88E: Calculate the equilibrium concentration of Zn2+ in a solution initially with 0.150 M Zn2+ and 2.50 M... Problem 89E: Calculate the Fe3+ equilibrium concentration when 0.0888 mole of K3[Fe(CN)6] is added to a solution... Problem 90E: Calculate the CO2+ equilibrium concentration when 0.100 mole of [CO(NH3)6](NO3)2 is added to a... Problem 91E: The equilibrium constant for the reaction Hg2+(aq)+2Cl(aq)HgCl2(aq) is 1.61013, Is HgCl2 a strong... Problem 92E: Calculate the molar solubility of Sn(OH)2 in a buffer solution containing equal concentrations of... Problem 93E: Calculate the molar solubility of Al(OH)3 in a buffer solution with 0.100 M NH3 and 0.400 M NH4+. Problem 94E: What is the molar solubility of CaF2 in a 0.100-M solution of HF? Ka for HF=7.2104. Problem 95E: What is the molar solubility of BaSO4 in a 0.250-M solution of NaHSO4? Ka for HSO4=1.2102. Problem 96E: What is the molar solubility of Tl(OH)3 in a 0.10-M solution of NH3? Problem 97E: What is the molar solubility of Pb(OH)2 in a 0.138-M solution of CH3NH2? Problem 98E: A solution of 0.075 M CoBr2 is saturated with H2S([H2S]=0.10M). What is the minimum pH at which CoS... Problem 99E: A 0.125-M solution of 0.075 Mn(NO3)2 is saturated with H2S([H2S]=0.10M). At what pH does Mns begin... Problem 100E: Calculate the molar solubility of BaF2 in a buffer solution containing 0.20 M HF and 0.20 M NaF. Problem 101E: Calculate the molar solubility of CdCO3 in a buffer solution containing 0.115 M Na2CO3 and 0.120 M... Problem 102E: To a 0.10-M solution of Pb(NO3)2 is added enough HF(g) to make [HF]=0.10M. (a) Does PbF2 precipitate... Problem 103E: Calculate the concentration of Cd2+ resulting from the dissolution of CdCO3 in a solution that is... Problem 104E: Both AgCl and Agl dissolve in NH3.. (a) What mass of AgI dissolves in 1.0 L of 1.0 M NH3?. (b) VNhat... Problem 105E: Calculate the volume of 1.50 M CH3CO2H required to dissolve a precipitate composed of 350 mg each of... Problem 106E: Even though Ca(OH)2 is an inexpensive base, its limited solubility restricts its use. What is the pH... Problem 107E: What mass of NaCN must be added to 1 L of 0.010 M Mg(NO3)2 in order to produce the first trace of... Problem 108E: Magnesium hydroxide and magnesium citrate function as mild laxatives when they reach the small... Problem 109E: The following question is taken from a Chemistry Advanced Placement Examination and is used with the... Problem 110E: Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility... Problem 111E: Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility... Problem 112E: What is the effect on the amount of solid Mg(OH)2 that dissolves and the concentrations of Mg2+ and... Problem 113E: What is the effect on the amount of CaHPO4 that dissolves and the concentrations of Ca2+ and HPO4-... Problem 114E: Identify all chemical species present in an aqueous solution of Ca3(PO4)2 and list these species in... Problem 115E: A volume of 50 mL of 1.8 M NH3 is mixed with an equal volume of a solution containing 0.95 g of... Problem 61E: Under what circumstances, if any, does a sample of solid AgCl completely dissolve in pure water?
Related questions
Based upon the chemical equilibrium reaction shown below explain what will happen in each scenario and why?
Transcribed Image Text: [Co(H2O)6]?* + 4 CI 2 [CoCli]? + 6 H2O
a.) 2 M HCI is added to the solution.
b.) Deionized water is added to the solution.
c.) If the solution turned blue upon heating in a 80°C water bath.
d.) If the solution turned Pink upon immersion into a 0° water bath.
Definition Definition State where the components involved in a reversible reaction, namely reactants and product, do not change concentration any further with time. Chemical equilibrium results when the rate of the forward reaction becomes equal to the rate of the reverse reaction.
Expert Solution
According to Le Chatelier’s principles, if there is a change in concentration, pressure, temperature, inert gases that affect equilibrium are changed, the equilibrium will shift in that direction where the effects of changes are nullified.
It is given that the following chemical reaction is in an equilibrium state
Step by step
Solved in 2 steps with 1 images
UNLOCK THE REST
![[Co(H2O)6]?* + 4 CI 2 [CoCli]? + 6 H2O
a.) 2 M HCI is added to the solution.
b.) Deionized water is added to the solution.
c.) If the solution turned blue upon heating in a 80°C water bath.
d.) If the solution turned Pink upon immersion into a 0° water bath.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Ff7a29198-be07-4c29-b139-38f759b3ffe2%2Fe105bfe0-1dab-422e-9213-16a05a66c04c%2Fjpcg12f_processed.png&w=3840&q=75)