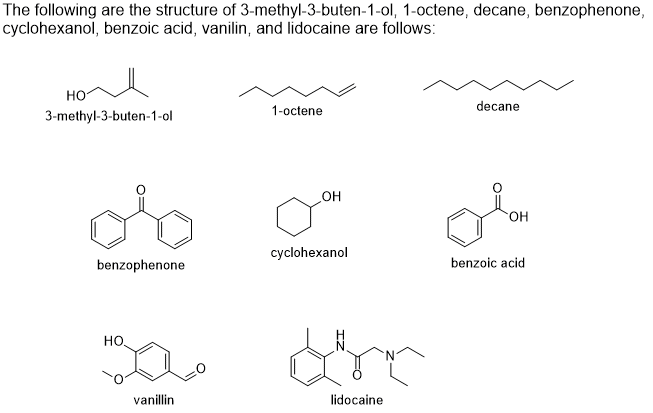
Based on the IR spectrscopy, identify if the solid compound is 3-methyl-3-buten-1-ol, 1-octene, decane, benzophenone, cyclohexanol, benzoic acid, vanilin, or lidocaine?
Based on the IR spectrscopy, identify if the solid compound is 3-methyl-3-buten-1-ol, 1-octene, decane, benzophenone, cyclohexanol, benzoic acid, vanilin, or lidocaine?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Based on the IR spectrscopy, identify if the solid compound is 3-methyl-3-buten-1-ol, 1-octene, decane, benzophenone, cyclohexanol, benzoic acid, vanilin, or lidocaine?

Transcribed Image Text:This image is a hand-drawn infrared (IR) spectroscopy graph representing transmittance as a function of wavenumber, measured in cm\(^{-1}\).
**Axis Descriptions:**
- **Vertical Axis:** Labeled as "Transmittance %", indicating the percentage of infrared light passing through a sample.
- **Horizontal Axis:** Labeled as "cm\(^{-1}\)", depicting the wavenumber, which is inversely proportional to wavelength.
**Graph Details:**
- The graph features multiple downward peaks, which represent the absorbance of specific wavelengths of infrared light by the sample molecules.
- Notable regions and peaks are observed near:
- 3500 cm\(^{-1}\)
- 3000 cm\(^{-1}\)
- 1500 cm\(^{-1}\)
- 1000 cm\(^{-1}\)
Each peak corresponds to a vibrational transition in the molecules, often associated with different types of bonds or functional groups in the sample. For example, peaks in the region of 3500 cm\(^{-1}\) are typically associated with N-H or O-H bond stretches.
This type of graph is commonly used in chemistry to identify functional groups within organic compounds. Understanding the significance of each peak is essential for interpreting the molecular structure and behavior of the sample.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY