balanced molecular equation (ME) write the balanced complete ionic equation (CIE) and write the balanced net ionic equation (NIE) Tin(II) chloride + potassium nitrate SnCl2(aq) KNO3(aq) Observations: No observable change; solution mixture is clear and colorless ME:
balanced molecular equation (ME)
write the balanced complete ionic equation (CIE)
and write the balanced net ionic equation (NIE)
Tin(II) chloride + potassium nitrate
SnCl2(aq) KNO3(aq)
|
||
|
||
|
||
|
The balanced molecular equation represents the non-dissociated molecular species participating in the chemical reaction. The balanced molecular equation for the given chemical reaction can be written as follows:
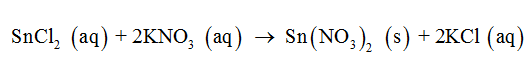
The balanced complete ionic equation represents the dissociated ions released from the molecules participating in the chemical reaction. The pure solids and liquids cannot be dissociated into their individual ions. The complete ionic equation for the given reaction can be written as follows:
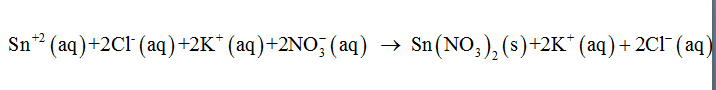
Step by step
Solved in 3 steps with 3 images









