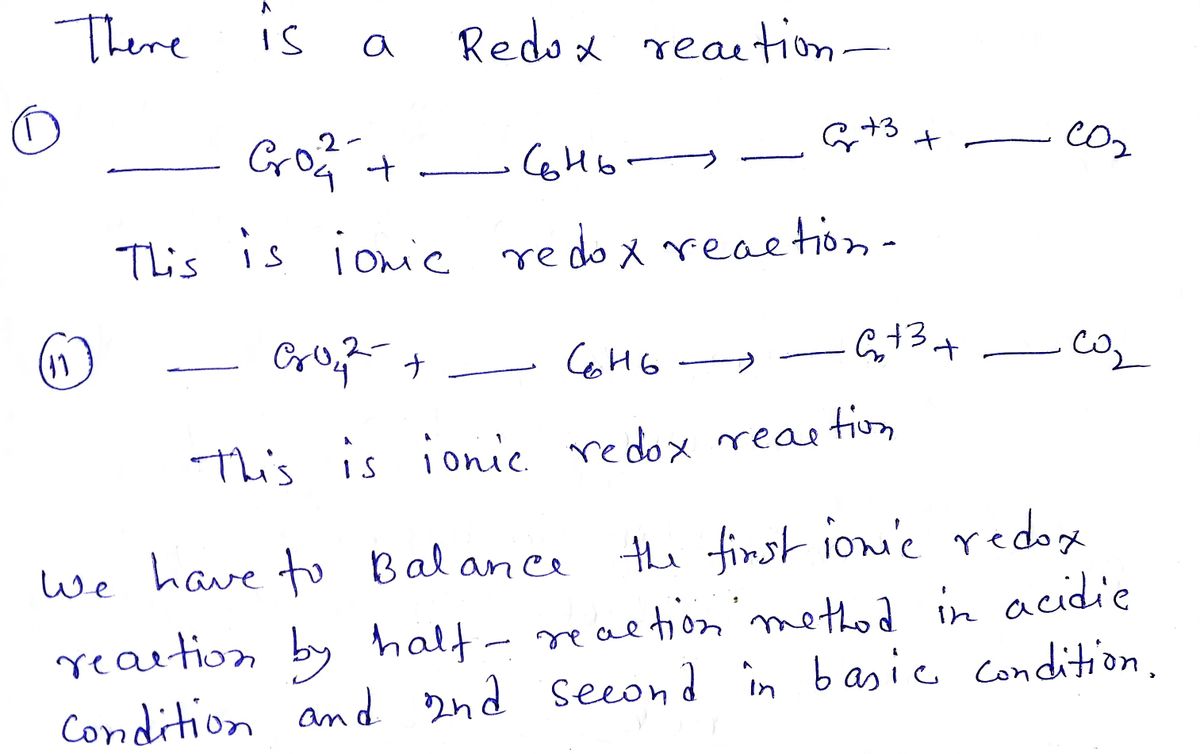
Balance the net ionic redox equations by the half-reaction method: full reaction balanced in acid: CrO42- + C6H6 Cr3+ +_CO2 reductant half-reaction: oxidant half-reaction:
Balance the net ionic redox equations by the half-reaction method: full reaction balanced in acid: CrO42- + C6H6 Cr3+ +_CO2 reductant half-reaction: oxidant half-reaction:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Balancing Net Ionic Redox Equations Using the Half-Reaction Method**
**Objective:**
Balance the following net ionic redox equations by the half-reaction method.
**Equation 1: Full Reaction Balanced in Acid**
\[
\_\_ \text{CrO}_4^{2-} + \_\_ \text{C}_6\text{H}_6 \rightarrow \_\_ \text{Cr}^{3+} + \_\_ \text{CO}_2
\]
**Steps:**
1. **Reductant Half-Reaction:**
- Identify and balance the reduction process.
2. **Oxidant Half-Reaction:**
- Identify and balance the oxidation process.
**Equation 2: Full Reaction Balanced in Base**
\[
\_\_ \text{CrO}_4^{2-} + \_\_ \text{C}_6\text{H}_6 \rightarrow \_\_ \text{Cr}^{3+} + \_\_ \text{CO}_2
\]
**Instructions:**
- Use the half-reaction method to balance each redox process in acidic and basic conditions.
- Adjust coefficients for atoms, charge, and electrons to achieve balance.
This educational guide focuses on teaching the half-reaction method, a vital technique in chemistry for balancing complex redox reactions.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc5b78197-8243-414c-9f1e-b207ae922b69%2F8d77d962-771d-47f9-9ed1-2ad2b197ac22%2Ffcd16v_processed.png&w=3840&q=75)
Transcribed Image Text:**Balancing Net Ionic Redox Equations Using the Half-Reaction Method**
**Objective:**
Balance the following net ionic redox equations by the half-reaction method.
**Equation 1: Full Reaction Balanced in Acid**
\[
\_\_ \text{CrO}_4^{2-} + \_\_ \text{C}_6\text{H}_6 \rightarrow \_\_ \text{Cr}^{3+} + \_\_ \text{CO}_2
\]
**Steps:**
1. **Reductant Half-Reaction:**
- Identify and balance the reduction process.
2. **Oxidant Half-Reaction:**
- Identify and balance the oxidation process.
**Equation 2: Full Reaction Balanced in Base**
\[
\_\_ \text{CrO}_4^{2-} + \_\_ \text{C}_6\text{H}_6 \rightarrow \_\_ \text{Cr}^{3+} + \_\_ \text{CO}_2
\]
**Instructions:**
- Use the half-reaction method to balance each redox process in acidic and basic conditions.
- Adjust coefficients for atoms, charge, and electrons to achieve balance.
This educational guide focuses on teaching the half-reaction method, a vital technique in chemistry for balancing complex redox reactions.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY