B) 95 x10³ C) 143x10 D) 303 x10 20) A particular vinegar solution has a H₂O* concentration of 0.013 M. What is the pOH of the solution? A) 17.3 B) 12.1 C) 12.3 D) 15.8
Ionic Equilibrium
Chemical equilibrium and ionic equilibrium are two major concepts in chemistry. Ionic equilibrium deals with the equilibrium involved in an ionization process while chemical equilibrium deals with the equilibrium during a chemical change. Ionic equilibrium is established between the ions and unionized species in a system. Understanding the concept of ionic equilibrium is very important to answer the questions related to certain chemical reactions in chemistry.
Arrhenius Acid
Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions. Keeping it similar to the general acid properties, Arrhenius acid also neutralizes bases and turns litmus paper into red.
Bronsted Lowry Base In Inorganic Chemistry
Bronsted-Lowry base in inorganic chemistry is any chemical substance that can accept a proton from the other chemical substance it is reacting with.
![11) Calculate the osmotic pressure associated with 90.0 g of an enzyme of molecular weight 98,000 g/mol |
dissolved in 2700 mL of benzene at 30.0 °C.
A) 5.99 torr
B) 1.96 torr
4
C) 6.43 torr
D) 2.48 torr
12) The rate constant for the first order reaction A--> B+C is k-4.4 x 103 min¹ at 57 K. What is the
half-life for this reaction at 57 K?
A) 32 min
C) 9.1 min.
D) 1200 min
13) Consider the following reaction:
If the half-life for the reaction is 0.610 s, the rate constant is:
2 N₂O(g) 2 NO(g) + O2(g); rate = k[N20]
A) 1.14 s¹¹
B) 0.69 ¹
C) 0.86 s¹
B) 16 min.
D) 0.32 s¹
14) The decomposition of N₂Os(g)-NO2(g) + NO(g) proceeds as a first order reaction with a half-life of
30.0 seconds at a certain temperature. If the initial concentration [N2Oslo 0.600 M, what is the
concentration after 120 seconds?
A) 0.038 M
B) 0.100 M
C) 0.400 M
D) 0.013 M
15) Which equation below best gives the concentration of N₂Os versus time in the previous question Q14?
A) [N₂0s] =([N₂Oslo)/t12 B) [N₂0s]-[N₂O3]oek
C) [N₂O₁]=kt D) 1/[N2Os] = 1/[N2Os]o + kt
16) Given: N2(g) + 3 H₂(g) <-> 2NH₂(g)
At equilibrium at a certain temperature, the concentration of NH3(g), H₂(g) and N2(g) are 0.49 M, 1.61 M and
0.14 M, respectively. Calculate the value of Ke for this reaction.
A) 1.26
B) 0.612
D) 1.96
C) 0.411
17) Consider the following reaction: 4 PCI(g)-> P4(g) + 6 Cl₂(g)
If the initial concentration of PCI3(g) is 2.0 M, and "x" is the equilibrium concentration of P4(g), what is the
correct equilibrium relation?
A) Kc = 6x7
B) Ke=6x7/(2.0-x)* C) Ke=x²/(2.0-x)*
18) Consider the reaction represented by the equation: N₂(g) + 3H2(g) <-> 2NH3(g) Calculate the
D) Kc = (x)(6x)/(2.0-4x)"
equilibrium pressures at a certain temperature: PNH₁-2.9 x 10 atm, PN2-89x10¹ atm, PH2-2.7x 10 atm
A) 4.9 x10
B) 2.9x10
D) 7.8x104
C) 6.3x104
55
19) Consider the reaction represented by the equation: Fe" (aq) + SCN (aq) →
FeSCN²+ (aq)
6.00 M Fe³+ (aq) and 10.0 M SCN (aq) are mixed at a certain temperature and at equilibrium
the concentration of FeSCN³+ (aq) is 3 M.
A) 250x10¹
B) 95 x103
20) A particular vinegar solution has a H3O* concentration
A) 17.3
B) 12.1
C) 143x103
D) 303 x10
of 0.013 M. What is the pOH of the solution?
D) 15.8
C) 12.3
21) What is the pH of 42.0 M aqueous solution of NH3 (ammonia). It's Ks- 1.8 x 10³.
A) 12.4
B) 0.0104
C) 0.778
22) The OH concentration in a 0.089M of Ca(OH)2 solution is
A) 0.178 M
B) 0.108 M
D) 1x 10-7 M
23) A solution of 9.00 MHCHO2 is 0.77% ionized in water. Calculate the K. value for the acid (HCHO₂).
A) 2.3x104
B) 5.4x104
C) 0.2x10³
D) 0.23
24) When a weak acid is titrated with a strong base, the pH at the equivalence point is ALWAYS
A) 7
B)<7
C) >7
D) < 1
C) 1.3x10-¹2 M
D) 12.6
25) Which one of these statements about strong acid is true
A) Strong acid produce solutions with a higher pH than weak acids
B) All strong acids have H atom bonded to electronegative oxygen atom
C) Strong acids are very concentrated acids
D) Strong acids are 100% ionized in water](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fbfaea92a-9638-4015-ac2a-6c50f8cc86a5%2Fb897d551-ccab-4607-8303-0a0f16492249%2Fkp3evycb_processed.jpeg&w=3840&q=75)
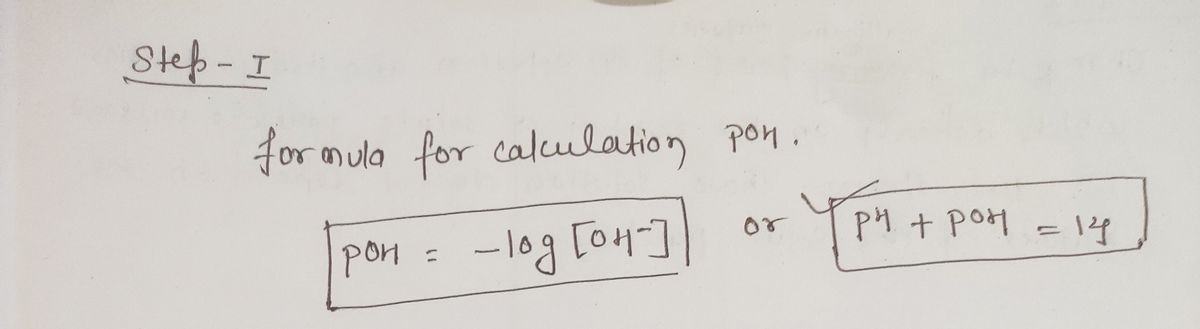
Step by step
Solved in 2 steps with 2 images









