At a certain temperature, the ?pKp for the decomposition of H2SH2S is 0.761.0.761. H2S(g)↽−−⇀H2(g)+S(g) Initially, only H2SH2S is present at a pressure of 0.151 atm0.151 atm in a closed container. What is the total pressure in the container at equilibrium?
At a certain temperature, the ?pKp for the decomposition of H2SH2S is 0.761.0.761.
Initially, only H2SH2S is present at a pressure of 0.151 atm0.151 atm in a closed container. What is the total pressure in the container at equilibrium?
consider the reaction:
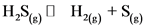
Initial partial pressure of 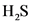 is 0.104 and equilibrium constant,
is 0.104 and equilibrium constant, 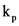 is 0.842
is 0.842
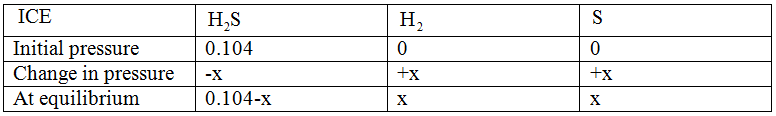
ICE table for the reaction is:
Use the expression:
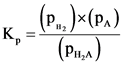
Substitute 0.842 for 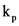 , x for
, x for 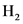 and x for
and x for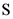
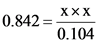
On solving, the equation is:
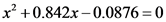
The value of x is solved by using the quadratic equation:
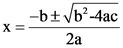
Substitute 0.842 for b, 1 for a and 0.0876 for c and solve the equation:
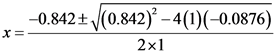
= 0.0936 or -0.936
The values for x is 0.0936
If x atm be the decrease in pressure of A at time t and one mole each of B and C is being formed, the increase in pressure of B and C will also be x atm.
As per the ICE table:
Equilibrium partial pressure of 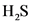 ,
, 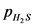 is 0.104 – x
is 0.104 – x
Substitute x as 0.0936
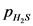 = 0.104 - 0.0936
= 0.104 - 0.0936
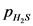 is 0.0104 atm.
is 0.0104 atm.
Equilibrium partial pressure of 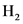 ,
, 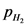 is x
is x
Substitute x as 0.0936
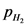 is 0.0936 atm
is 0.0936 atm
Equilibrium partial pressure of 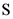 ,
, 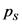 is x
is x
Substitute x as 0.0936
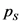 is 0.0936 atm
is 0.0936 atm
The value of x is 0.0936 which is used to calculate the equilibrium partial pressure of the gases.
Step by step
Solved in 2 steps with 27 images









