answer 5 of the 7 questions Draw and name the Stero chemical ISOmer 4-chlere- 1 methylcyclohexane which Chair conformation of has the mos c) Name the following molecule using R, S, absolute Configuration. H CI ||||| CH3 stable C43 f) Place the following molecules in increasing base strength (label the as 1) lowest Na OCH 3, KBr, NaH, H₂O, CH 3 CH ₂ NH ₂

Cyclohexane is a saturated cyclic compound with six carbon atoms on the ring. The most stable conformer of cyclohexane is the chair conformer. It has neither angle strain nor torsional strain, so cyclohexane exists in the chair conformation.
There are two kinds of bonds present in cyclohexane. They are axial bonds and equatorial bonds. The structure of cyclohexane with these bonds is shown below.
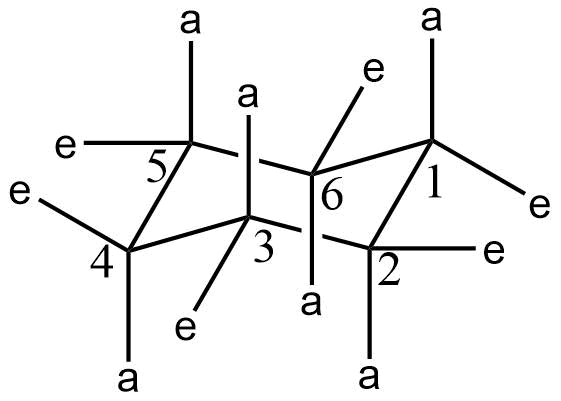
a represents the axial bond and e represents equatorial bonds.
A substituent is more stable at an equatorial position than at axial position.. Consider an axial substituent at C-1. This substituent can interact with an axial substituent or axial hydrogen of C-3. These kinds of interactions are called 1,3 dialkyl interactions. This interaction is generally repulsive and reduces the stability of compounds. So generally for substitutions, the equatorial position is preferred over the axial position.
Note: Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved then please specify the question number or post only that question.
Step by step
Solved in 5 steps with 5 images









