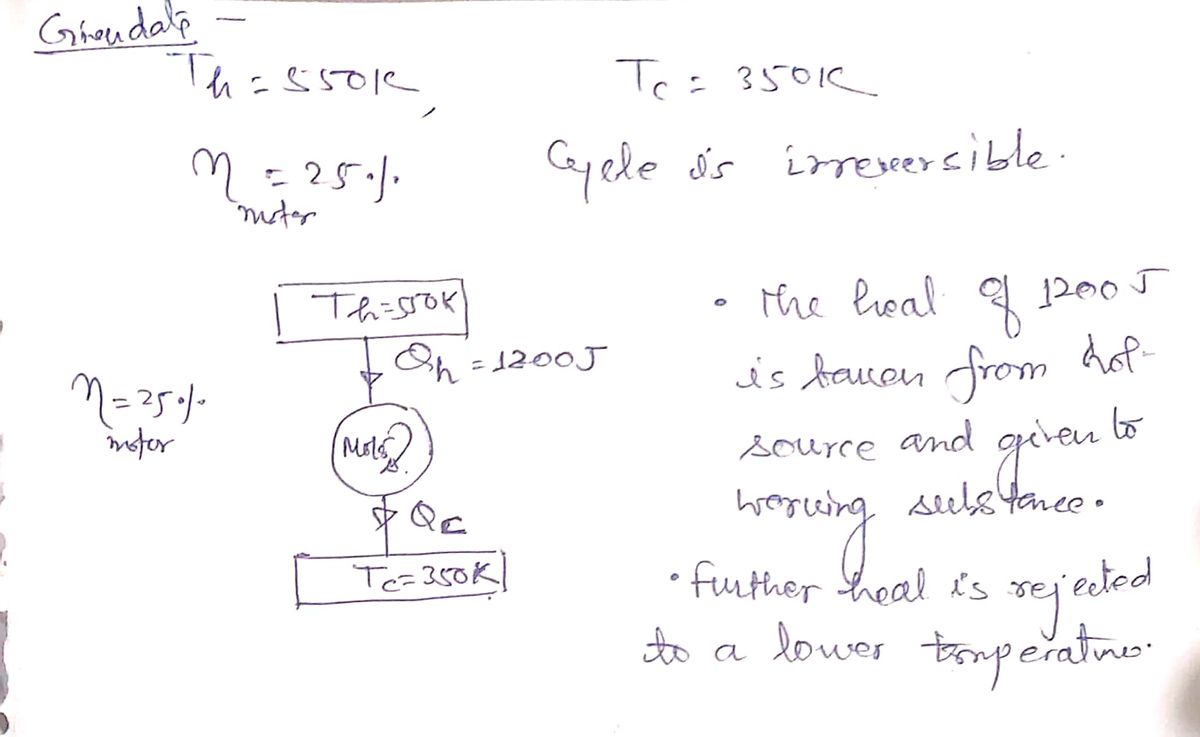
An irreversible motor operates between reservoirs at Th = 550 K and Tc = 350 K, with an efficiency of 25%. In each cycle heat Qh = 1200 J is extracted from the reservoir at temperature Th, and this heat is added to the working substance in the motor. The heat |Qc| is taken from the motor to the reservoir at temperature Tc. A) Determine the change in entropy of the universe for one cycle of operation B) How much more work could be done by a reversible motor operating between these reservoirs, with the same heat input for each cycle? Justify C) Show that for each cycle, the amount of work that becomes unavailable due to the irreversibility of the process is equal to TcΔSuniverse
An irreversible motor operates between reservoirs at Th = 550 K and Tc = 350 K, with an efficiency of 25%. In each cycle heat Qh = 1200 J is extracted from the reservoir at temperature Th, and this heat is added to the working substance in the motor. The heat |Qc| is taken from the motor to the reservoir at temperature Tc. A) Determine the change in entropy of the universe for one cycle of operation B) How much more work could be done by a reversible motor operating between these reservoirs, with the same heat input for each cycle? Justify C) Show that for each cycle, the amount of work that becomes unavailable due to the irreversibility of the process is equal to TcΔSuniverse
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
An irreversible motor operates between reservoirs at Th = 550 K and Tc = 350 K, with an efficiency of 25%. In each cycle heat Qh = 1200 J is extracted from the reservoir at temperature Th, and this heat is added to the working substance in the motor. The heat |Qc| is taken from the motor to the reservoir at temperature Tc.
A) Determine the change in entropy of the universe for one cycle of operation
B) How much more work could be done by a reversible motor operating between these reservoirs, with the same heat input for each cycle? Justify
C) Show that for each cycle, the amount of work that becomes unavailable due to the irreversibility of the process is equal to TcΔSuniverse
Expert Solution
Step 1
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY