An ice tray contains 360 g of liquid water at 0°C. Calculate the change in entropy of the water as it freezes slowly and completely at 0°C.
Q: What is the change in entropy as 58.3 J flows from 331°C to 197°C?
A: Given: Q=58.3j T1=331 deg.c=604K T2=197 deg.c=470K Lets's find Change in entropy.
Q: The radiator of a steam heating system has a volume of 20 L and is filled with superheated water…
A: It is given that,
Q: Steam initially at 100° C is mixed with 150 g of ice at -60.0° C, in a thermally insulated…
A:
Q: A standard basketball has a mass of 1.4 pounds. This basketball is lifted (3 + y/20) ft above the…
A: Write the given values of this problem. m=1.4 poundsm=1.4×0.45359237m=0.635 kgh=3+y20…
Q: Humans cool off by perspiring; the evaporating sweat removes heat from the body. If the skin…
A: The objective of the question is to calculate the entropy change of the universe due to the…
Q: Calculate the change in entropy when the pressure of an ideal gas is changed isothermally from Pi to…
A:
Q: 500 g of ice at -10.0°C are placed on top of a 2.00 kg slab of aluminum at 30.0°C. The system is…
A:
Q: Consider taking two identical ice cubes out of the freezer. You place one in a cup and let it melt;…
A: Given Two identical ice cubes out of the freezer, You place one in a cup and let it melt; you take…
Q: A heat engine absorbs 2500 J of heat at 250°C and expels 2000 J at a temperature of 50°C. The change…
A: Given: Heat absorbed at T1=250°C is Q1=2500 J Heat expelled at T2=50°C is Q2=2000 J
Q: Two moles of an ideal gas have an initial Kelvin temperature T; and absolute pressure P₁. The gas…
A:
Q: A freezer is used to freeze 1.5 L of water completely into ice. The water and the freezer remain at…
A: The expression for change in entropy is, ∆S=QT…
Q: A 10 g ice cube at -10C is placed in a lake whose temperature is 15C. Calculate the change in…
A:
Q: Every second at Niagara Falls, approximately 5.00 x 103 m3 of water falls a distance of 50.0 m. What…
A:
Q: On a hot summer day, 4.00×106J of heat transfer into a parked car takes place, increasing its…
A: The expression used to find average temperature is T=T1+T22 Insert values in the above equation…
Q: A 5 kg block of ice initially at 268.15 K is placed in thermal contact with a very large reservoir…
A:
Q: The surface of the Sun is approximately at = 700 K, and the temperature of the Earth’s surface is…
A:
Q: 7 kg of water at 55oC is added to a large quantity of ice at 0oC resulting in ice-water. What is the…
A:
Q: What is the entropy change of 15 g of steam at 100°C when it condenses to water at the same…
A: Part ASolution:Entropy change will be given as:dS = QT - 1where, Q is the heat amount of…
Q: 45-g of water at 14°C is heated until it becomes vapor at 100°C. Calculate the change in entropy of…
A: Given, Mass=45g Initial Temperature=14degree Final temparature=100degree
Q: One container holds 0.10 kg of water at 80 C and is warmed to 100 C by heating from contact with the…
A:
Q: Two 1100-kg cars are traveling 85 km/h in opposite directions when they collide and are brought to…
A: According to the given data, Mass of the cars (M) = 1100 kg; Speed of the car (v) = 85 km/h = 23.61…
Q: 170 g of water at 105°C is converted to steam. What is the entropy change of the water that is now…
A: The expression for find change in entropy is ∆s=mLvT
Q: A glass of 450.0 g water at 20°C is chilled using a 20.0 g icecube at 0°C. Assume that the mixing…
A:
Q: Calculate the change in entropy of 290 g of water warmed slowly from 25.0°C to 80.0°C. 1/K
A: Given Data The mass of the water is given as m = 290gm. The initial temperature of the water is…
Q: A puddle containing 1.10 kg of water at 0°C freezes on a cold night, becoming ice at 0°C. What was…
A: Given that The mass of the water 1.10Kg covert into gram m = 1100g Latent…
Q: A cylinder-piston system with 700 g of water at 0C is placed in a refrigerator. Water slowly freezes…
A: Given data The mass of the water is mw = 700 g = 0.7 kg The initial temperature of the water is Ti_w…
Q: A tank of 100-g liquid water at 100°C is put in thermal contact with another tank of 300-g water at…
A: mass of water in first tank = 100 g Initial temperature of water in first tank = 100oC mass of water…
Q: A cylinder of gas has a temperature of 32F. a) If 205J is added to the system at constant…
A: Given Gas is at T=32oF=273.15K a) The amount of heat added to the system is Q=205J So at a…
Q: A 2.00-L container has a center partition that divides it into two equal parts as shown. The left…
A: The gases given are at room temperature. The partition is removed and gases are allowed to mix. It…
Q: A 2.8 kg piece of aluminum at 43.0 degrees C is placed in 1.0 kg of water in a Styrofoam container…
A:

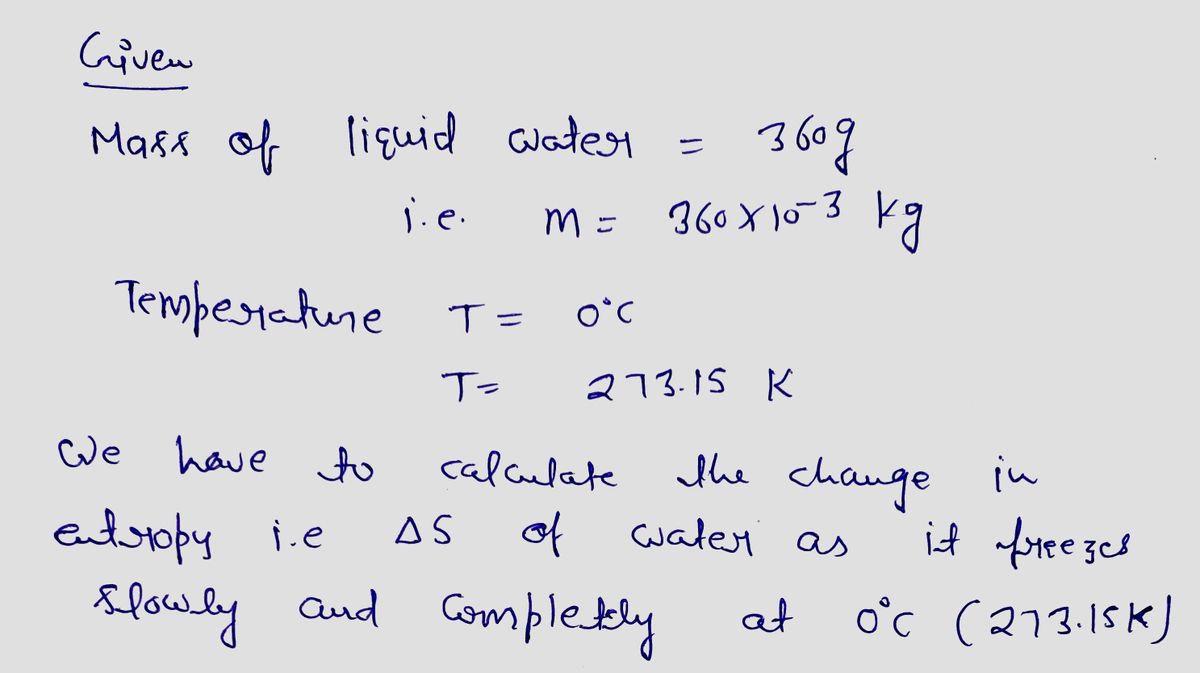
Step by step
Solved in 2 steps with 2 images

- A 1 500-kg car is moving at 20.0 m/s. The driver brakes to a stop. The brakes cool off to the temperature of the surrounding air, which is nearly constant at 20.0°C. What is the total entropy change?What is the change in entropy in converting 1.0 kg of ice at 0.0 degree celsius to 1.0 kg water at 0.0 degree celsius?A 2.00-L container has a center partition that divides it into two equal parts as shown below. The left side contains n = 0.061 0 mol of H, gas, and the right side contains n = 0.061 0 mol of 0, gas. Both gases are at room temperature and at atmospheric pressure. The partition is removed, and the gases are allowed to mix. What is the entropy increase of the system? וןנ
- Suppose that when you exercise, you consume 100g of glucose and that all the energy released through the mechanism of heat remains in your body at 37 degrees Celsius. What is the change in entropy of your body? (ans: 5.03 kJ/K)A room is at a constant 295 K maintained by an air conditioner that pumps heat out. How much does the refrigerator change the entropy AS of the room for each 5.80 kJ of heat that it removes? AS = J/KA 100-lbm block of a solid material whose specific heat is 0.5 Btu/lbm·R is at 80°F. It is heated with 10 lbm of saturated water vapor that has a constant pressure of 20 psia. Determine the final temperature of the block and water, and the entropy change of the entire system. Is this process possible? Why?