Air Air V= constant T = 20°C T = 30°C P= constant T= 20°C T= 30°C Au = cyAT = 7.18 kJ/kg Au = c,AT = 7.18 kJ/kg
The relation Δu = cv ΔT is valid for any kind of process, constant-volume or not.

Given data:
*The initial temperature of the first system is T1i=20°.
*The final temperature of the first system is T1f=30°.
*The volume is constant for first process.
*The initial temperature of the second system is T2i=20°.
*The final temperature of the second system is T2f=30°.
*The pressure is constant for first process.
According to the first law of thermodynamics the change in internal energy of the system is given by:
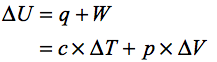
Here, c is the specific heat capacity of the air.
In the first case the volume of the system is constant thus, the work done at constant volume is zero hence the change in internal energy is only due to the heat transfer.Thus,
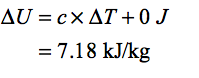
Step by step
Solved in 2 steps with 2 images









