Determination of a Metal What are the different copper compounds that has ≥30% Copper composition? list down with the exact % Copper composition.
Determination of a Metal What are the different copper compounds that has ≥30% Copper composition? list down with the exact % Copper composition.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Determination of a Metal
What are the different copper compounds that has ≥30% Copper composition? list down with the exact % Copper composition.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
According to the first image, if an unknown copper metal compound, has 37.2% Cu then it is most likely a Copper sulfate. If you compare the %Cu using the 2nd image though, Copper acetate has a closer %Cu value. Why is it Copper sulfate then?
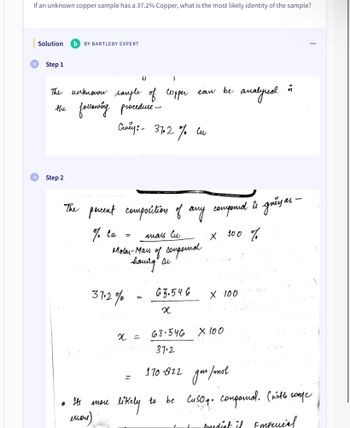
Transcribed Image Text:If an unknown copper sample has a 37.2% Copper, what is the most likely identity of the sample?
Solution Ь BY BARTLEBY EXPERT
Step 1
can
аналунов
е ѝ
the
The unknoow sample ов соррес как ве
following procedure -
Смеу: - 3702 % си
Step 2
percent composition of any compound is grey as -
% си
=
лиац Си
х 100 %
моли-мац од сопротое
having an
въ.546
=
X 100
х
X 100
х =
63.546
37.2
S
170 822 дом/mol
/тное
likely
to be CuSO4. companol. (with some
boudist if Emperial
The
37.2%
Its mor
мой)
Transcribed Image Text:SEARCH
ASK AN EXPERI
CHAI VX MATH SOLVER
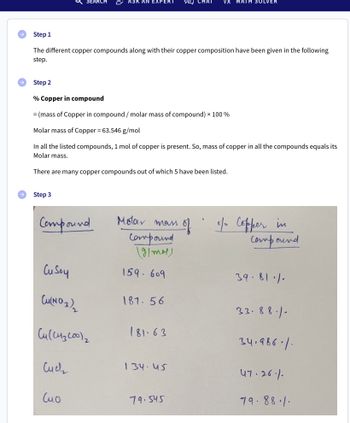
Step 1
The different copper compounds along with their copper composition have been given in the following
step.
Step 2
% Copper in compound
= (mass of Copper in compound / molar mass of compound) x 100 %
Molar mass of Copper = 63.546 g/mol
In all the listed compounds, 1 mol of copper is present. So, mass of copper in all the compounds equals its
Molar mass.
There are many copper compounds out of which 5 have been listed.
Step 3
Molar mass
Compound
of of Copper in
Compound
(g/mol)
Cu Soy
Cu(NO3)₂
Cu (CH3200) ₂
Cuch
Сио
159.609
187.56
181-63
134.45
79.545
compound
39.81.1.
33.88.1-
34.986./
47.26.1.
79.88.1.
Solution
Follow-up Question
If an unknown copper sample has a 37.2% Copper, what is the most likely identity of the sample?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY