a. What is the mass in grams of 5.01 x 1023 a. What is the mass in grams of 5.01 × 10° atoms of lead? grams b. How many lead atoms are there in 49.3 grams of lead? atoms
a. What is the mass in grams of 5.01 x 1023 a. What is the mass in grams of 5.01 × 10° atoms of lead? grams b. How many lead atoms are there in 49.3 grams of lead? atoms
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![### Chemistry Problem Set
**Question a:**
What is the mass in grams of \(5.01 \times 10^{23}\) atoms of lead?
- Answer: [ ] grams
**Question b:**
How many lead atoms are there in 49.3 grams of lead?
- Answer: [ ] atoms
**Submission Options:**
- Submit Answer
- Retry Entire Group
**Attempts Remaining:**
- 9 more group attempts remaining
(Note: Make sure to use the references provided to access important constants and conversion factors necessary for solving these problems.)](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe145b44d-a192-40af-a48f-7d24a9c43f47%2Ffce72bb7-d2de-4f64-b1cf-abac6944ff54%2Fmyc4hzm_processed.jpeg&w=3840&q=75)
Transcribed Image Text:### Chemistry Problem Set
**Question a:**
What is the mass in grams of \(5.01 \times 10^{23}\) atoms of lead?
- Answer: [ ] grams
**Question b:**
How many lead atoms are there in 49.3 grams of lead?
- Answer: [ ] atoms
**Submission Options:**
- Submit Answer
- Retry Entire Group
**Attempts Remaining:**
- 9 more group attempts remaining
(Note: Make sure to use the references provided to access important constants and conversion factors necessary for solving these problems.)
Expert Solution
Step 1
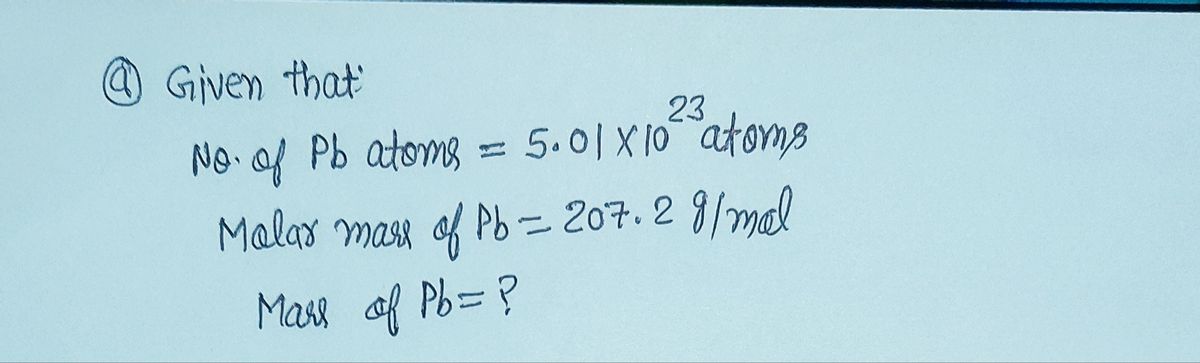
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY