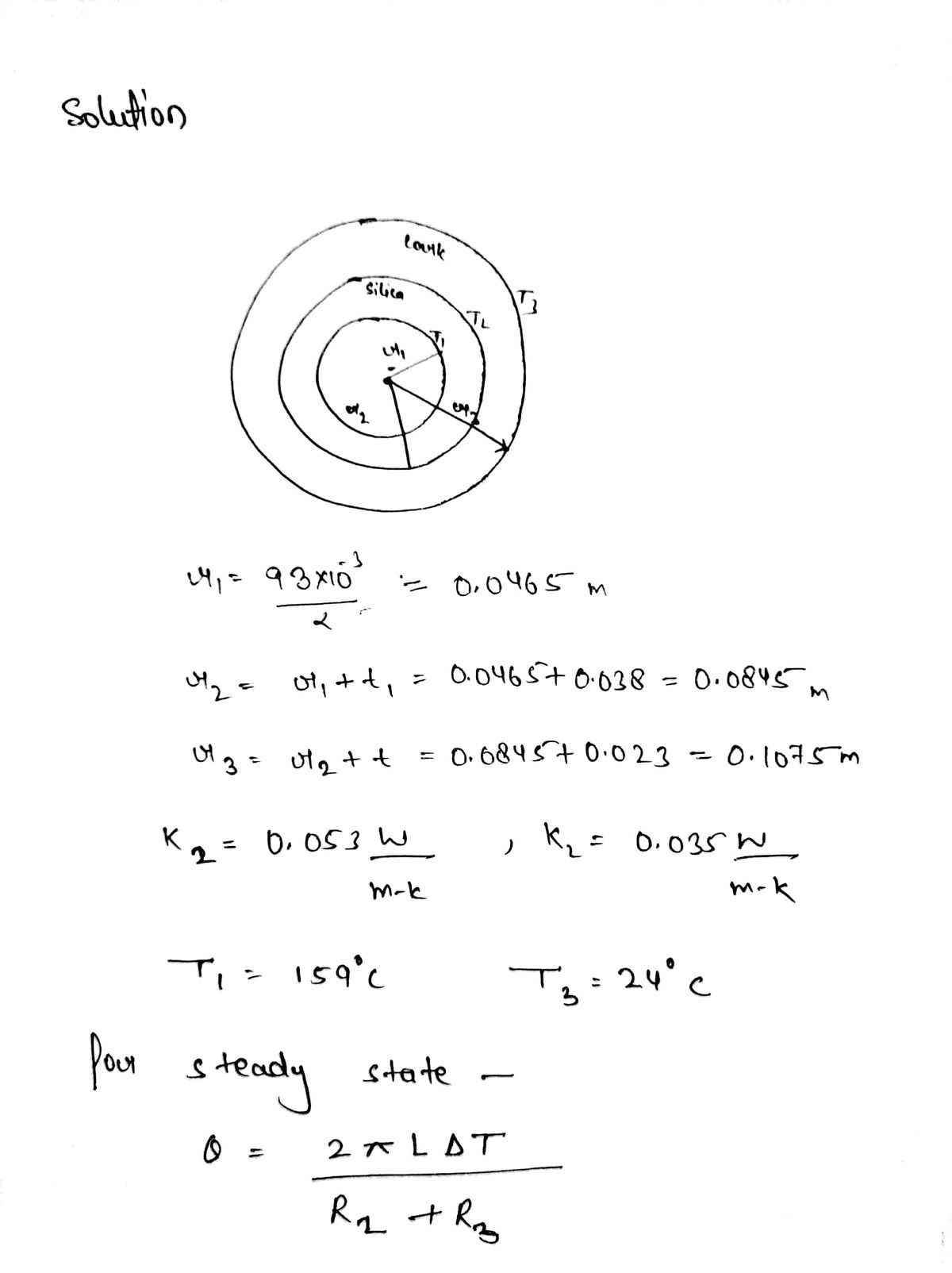
A tube 93 mm outer diameter is insulated with a layer of silica foam 38 mm thick of conductivity 0.053 W/m-K, followed by a 23 mm thick layer of cork of conductivity 0.035 W/m-K. If the temperature on the outer surface of the pipe is 159 °C and the temperature of the outer surface of the cork is 24 °C, calculate the heat loss per secon per metre of pipe across both layers.
A tube 93 mm outer diameter is insulated with a layer of silica foam 38 mm thick of conductivity 0.053 W/m-K, followed by a 23 mm thick layer of cork of conductivity 0.035 W/m-K. If the temperature on the outer surface of the pipe is 159 °C and the temperature of the outer surface of the cork is 24 °C, calculate the heat loss per secon per metre of pipe across both layers.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
I have included some formulas

Transcribed Image Text:A tube 93 mm outer diameter is insulated with a layer of silica foam 38 mm thick of
conductivity 0.053 W/m-K, followed by a 23 mm thick layer of cork of conductivity
0.035 W/m-K. If the temperature on the outer surface of the pipe is 159 °C and the
temperature of the outer surface of the cork is 24 °C, calculate the heat loss per second
per metre of pipe across both layers.

Transcribed Image Text:Cylindrical
Conduction
WITH
Convection
Cylindrical
Conduction
WITH
Convection
AND Fouling
Factors
Q=
Q:
Q
Q
1
U₂
2
d.h.
=
1
h₂ Vi
+
+
Do
Dh₂
h₂
1
+
2π Lt AT
In(d, /d.)
k
2π LtAT
In(D./D;)
k
+
D₂
D₂
Dhai Dh
+
2πr. LAT
D¡ln(D/D₂)
2k
D. 1
Dihai
+
2πr LAT
D.In(D/D) 1
2k
+
ho
+
+
+
2
d.h
1
h₂ro.
+
Di
Doho
2πr, LAT
D.In(D/D₂)
2k
Q=
2
d.h
1 1
+ +
+
ho nao.
2π Lt AT
ln(d/d) In(d/d.) 2
k₁
k₂
dh
If based on outside area, A.-2лr L. Same as
equation above on left but for one second. To get
above equation divide numerator and
denominator by r.)
If based on inside area, A-2πr;L Same as equation
above on left but for one second. To get above
equation divide numerator and denominator by r;)
U₂₁
+
+...+.
+
1
Do Do
x₂ Do
Dha Dh k
+
1
D. 1 (D₂/2){ln(D¸ /D;)} _ _1
+
+
+
Di Jh;
k
ho hao
1
+
no hio
{Either formula to get U。}
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The