A temperature rise of 3.10 ± 0.20 °C was measured when a reaction was carried out in a calorimeter with a heat capacity of 0.841 ± 0.014 kJ °C -1 . The enthalpy change (Δ H ) was worked out as -2.61 kJ by using the equation: Enthalpy change (Δ H ) = heat capacity ( c ) × temperature change ( T ) What is the root-squared error (in kJ) for Δ H ? Quote your answer to two decimal places. When you submit your answer, do NOT include units. (The minus sign in front of the enthalpy change indicates that the reaction is exothermic; don't include it in your calculation.)
A temperature rise of 3.10 ± 0.20 °C was measured when a reaction was carried out in a calorimeter with a heat capacity of 0.841 ± 0.014 kJ °C -1 .
The enthalpy change (Δ H ) was worked out as -2.61 kJ by using the equation:
Enthalpy change (Δ H ) = heat capacity ( c ) × temperature change ( T )
What is the root-squared error (in kJ) for Δ H ?
Quote your answer to two decimal places.
When you submit your answer, do NOT include units.
(The minus sign in front of the enthalpy change indicates that the reaction is exothermic; don't include it in your calculation.)
Write the given values using suitable variables.
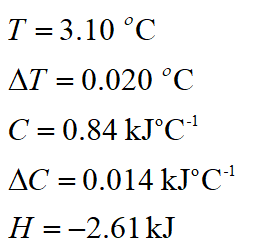
Here T signifies temp, C signifies heat capacity, and H signifies enthalpy change.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images









