A student determines the value of the equilibrium constant to be 7.94×10-37 for the following reaction. H2S(g) + 2H2O(l)3H2(g) + SO2(g) Based on this value of Keq: G° for this reaction is expected to be (greater, less) than zero. Calculate the free energy change for the reaction of 1.85 moles of H2S(g) at standard conditions at 298K. G°rxn = kJ
A student determines the value of the equilibrium constant to be 7.94×10-37 for the following reaction.
H2S(g) + 2H2O(l)3H2(g) + SO2(g)
Based on this value of Keq:
G° for this reaction is expected to be (greater, less) than zero.
Calculate the free energy change for the reaction of 1.85 moles of H2S(g) at standard conditions at 298K.
G°rxn = kJ
The given reaction is:
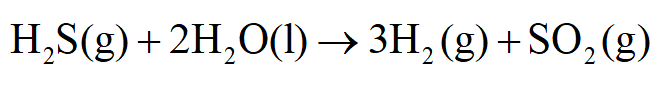
The equilibrium constant for this reaction, Keq is 7.94×10-37.
Since the value of Keq is very low(less than 1), thus the reaction will be non spontaneous. For a non spontaneous reaction, ∆G˚ is positive. Thus based on the value of Keq, ∆G˚ for this reaction will be greater than 0.
Calculation of ∆G˚ of this reaction at 298 K:
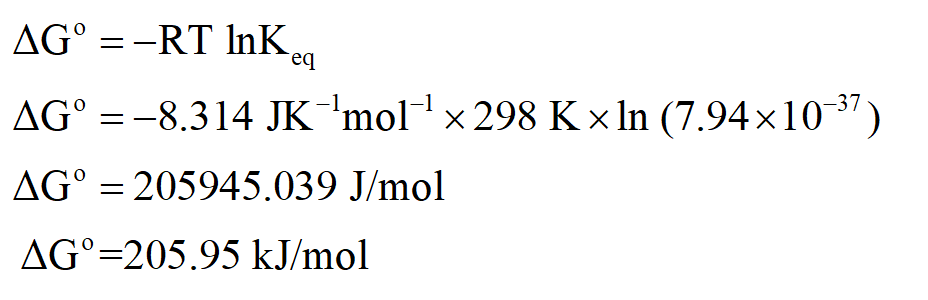
Thus ∆G˚ of this reaction will be greater than zero at equilibrium.
Step by step
Solved in 2 steps with 3 images









