A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ> Mega Joules) is transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects. kJ/kmol.K. The value of universal gas constant is 8.314
A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ> Mega Joules) is transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects. kJ/kmol.K. The value of universal gas constant is 8.314
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Qsafe = 187.586 MJ

Transcribed Image Text:2. A rigid storage tank contains 30 (kg) of ideal hydrogen (M=2.016 kg/kmol). During a fire, heat (128.5 MJ→ Mega Joules) is
transferred to the tank. The initial temperature and pressure are 300 (K) and 500 (kPa), respectively. The burst pressure of tank
is 1.5 MPa. Will the tank rupture? Ignore kinetic and potential effects.
kJ/kmol.K.
The value of universal gas constant is 8.314
Expert Solution
Step 1
The given data are:
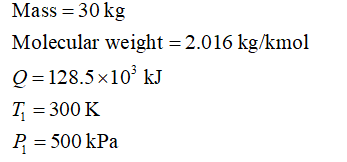
Step 2
As it mentions the tank is rigid, therefore the volume is constant.
Now apply the first law of thermodynamics.
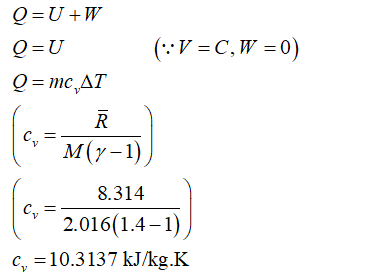
Step by step
Solved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY