A racing bicycle tire contains 0.65 moles of gas and has a volume of 2.8L at 295 K. What is the pressure in the tire in atmospheres? Remember to report your answer with the appropriate number of significant figures and a unit (abbreviated form ofbthe unit)
A racing bicycle tire contains 0.65 moles of gas and has a volume of 2.8L at 295 K. What is the pressure in the tire in atmospheres? Remember to report your answer with the appropriate number of significant figures and a unit (abbreviated form ofbthe unit)
____________
Ideal gas law is a law combined from various laws. In an ideal gas, the pressure is inversely proportional to volume. The volume of an ideal gas is directly proportional to temperature and number of moles of gas. From the above-stated law, the ideal gas equation is shown below.

Given,
Moles of gas= 0.65
Volume of gas = 2.8L
Temperature of gas = 295 K
Gas constant = 0.08205 L atm mol-1 K-1
Substitute the given values in above equation to get the pressure of gas in tire.
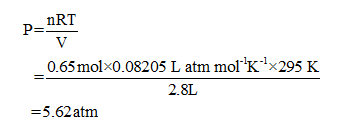
Step by step
Solved in 3 steps with 2 images









