A plate column as show in Figure Q4 is used to recover solute A from a dilute gas mixture using recycle water as solvent. The solvent entering flow rate is 280 kgmol/h and consists of 99.9 mol% of water and 0.1 mol% of A. The gas mixture enter the column with 6 mol% of solute A and flow rate of 150 kgmol/h. The required concentration of solute A in leaving stream, xA is 0.03. Assume the operating temperature and pressure are constant with the equilibrium relation for this system is у- 1.25х. L- 280 kgmol/h X= 0.001 L = 280 kgmol/h X = 0.03 V = 150 kgmol/h YA=0.06 Figure Q4: Gas absorption System (a) Determine the amount of streams leaving the column, L, and Vz. (b) Determine the number of ideal stages analytically. (c) Based on the minimum solvent flow rate, comment on the actual solvent flow rate for this system.

Plate column is used to recover solute from the dilute gas mixture using water as a solvent.
molar flowrate of entering solvent,
mole fraction of water in entering solvent,
mole fraction of solute A,
molar flowrate of entering gas mixture,
mole fraction of A in entering gas,
mole fraction of A in existing solvent,
The equilibrium relationship is given as,
...... (1)
(a)
The Schematic of the process is given as,
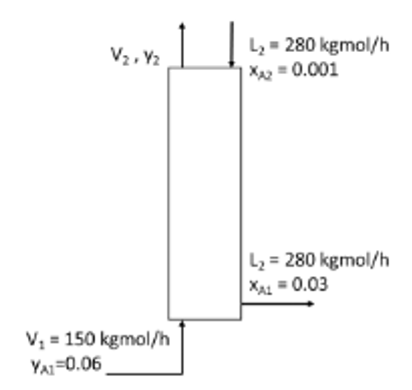
Write the overall material balance on the extraction column,
...... (2)
Substitute , , in equation (2)
Write the material balance of A in the column,
...... (3)
Substitute , , , , , , in equation (3)
Therefore, A in L1=
A in V2 =
Step by step
Solved in 4 steps with 1 images









