Predict the molecular geometry of each of the following
molecules:

(a)Given molecule is
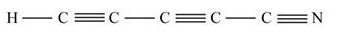
The shape of the molecule is determined by the geometry possess by the carbon atom present in the molecule.
From the given structure, it is clear that each carbon (C) atom has two electron domains which are bonding. Therefore, the geometry of electron domain is linear having sp hybridization. Hence, the molecule has linear shape.
(b)Given molecule is
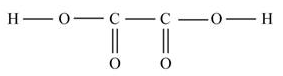
The shape of the molecule is determined by the geometry possess by the carbon atom present in the molecule.
From the given structure, it is clear that each carbon (C) atom at the center of the molecule has three electron domains which are bonding. Therefore, the geometry of electron domain is trigonal planar having sp2 hybridization. The carbon and oxygen atoms are arranged in the plane and H atoms are free to rotate in and out of the plane. Hence, the molecule has trigonal planar shape.
Step by step
Solved in 3 steps with 4 images









