A metal "X" is used as a “sacrificial” anode on hulls of salt water vessels made of iron. The effect is to reverse the oxidation of iron to iron (II) by causing X to oxidize to the X ion. Calculate the voltage (in V, to two decimal places) for this reaction if it takes place under standard conditions. The standard reduction potential for X is -1.20 V (minus 1.20 V).
A metal "X" is used as a “sacrificial” anode on hulls of salt water vessels made of iron. The effect is to reverse the oxidation of iron to iron (II) by causing X to oxidize to the X ion. Calculate the voltage (in V, to two decimal places) for this reaction if it takes place under standard conditions. The standard reduction potential for X is -1.20 V (minus 1.20 V).
The voltage of a reaction in the electrochemical cell is expressed in terms of the electrode potential of the cell. Electrode potential of the cell is given by the expression given in the equation (1) in which E0 is the electrode potential, E0cathode is the reduction potential of reduction half cell and E0anode is the reduction potential of oxidation half-cell.
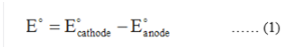
Step by step
Solved in 2 steps with 2 images









