A meat processing factory’s raw effluent has a five day BOD at 20oC of 1760 mg/l. If the k value is 0.15/day determine the BOD at two and four days at 20oC
A meat processing factory’s raw effluent has a five day BOD at 20oC of
1760 mg/l. If the k value is 0.15/day determine the BOD at two and four days at 20oC
Given data:
Five day BOD = 1760 mg/L
The value of k = 0.15/day
Temperature = 20 0C
A meat processing factory’s raw effluent has a five day BOD (biological oxygen demand).
The rate equation for BOD calculation follows the first order kinetics.
The kinetics equation for the first order reaction is,
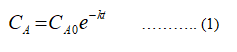
Where
CA represents concentration at time t of species A (BOD0).
CA0 represents initial concentration (t= 0) of species A (BODt).
k represents rate constant.
t represents time.
Rate constant is function of time. The value of rate constant is given at temperature of 20 0C.
The BOD to be calculated is also at the same temperature. Therefore, the value of rate constant will remains constant.
The ultimate BOD at five day is calculated as follows:
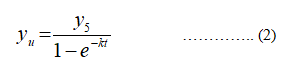
yu represents ultimate concentration.
y5 represents five day BOD (concentration).
Five day BOD = 1760 mg/L
The value of k = 0.15/day
Plugin the values in equation (2)
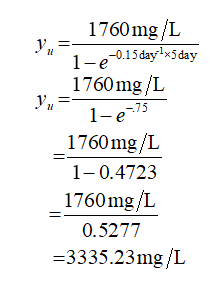
Step by step
Solved in 5 steps with 6 images









