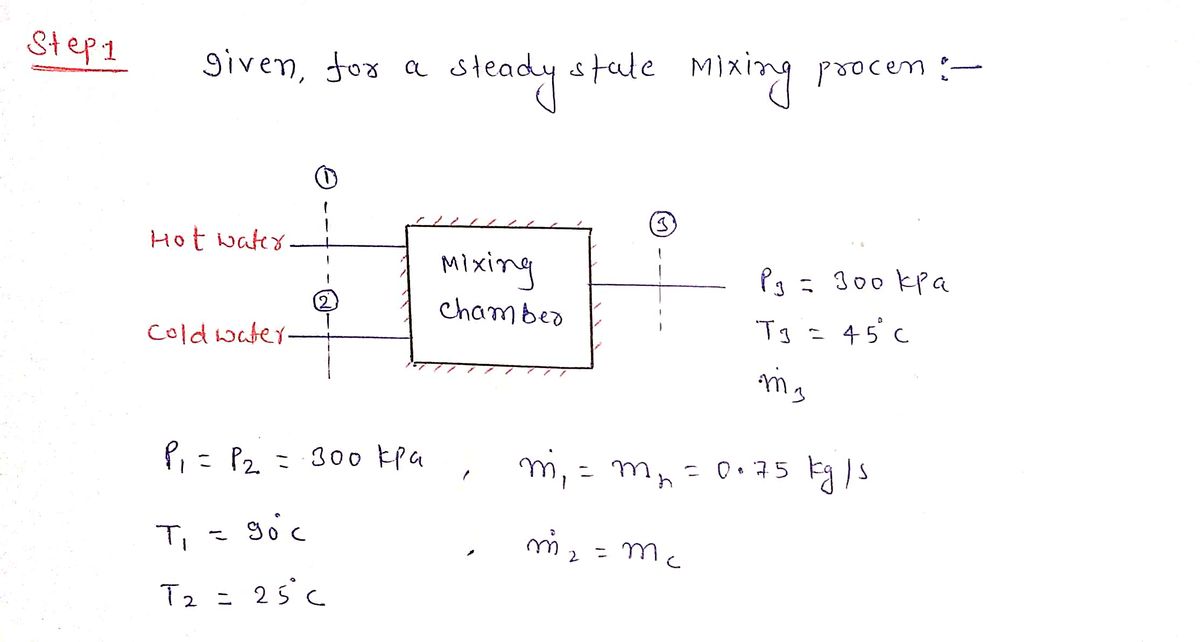
A hot-water stream at 90 °C enters an insulated mixing chamber with a mass flow rate of mh=0.75 kg/s where it is mixed with a stream of cold water at 25°℃ in a steady state steady flow process. It is desired that the mixture leaves the chamber at 45°C as shown in the Figure Hot water P₁=300 kPa T₁=90°C m=0.75 kg/s Cold water P: 300 kPa T:=25C m=? 2 Mixing Chamber 3 Mixed warm water Ps=300 kPa T3=45°C m3 For Question 1: The specific enthalpy of the hot water, h1, in kJ/kg is
A hot-water stream at 90 °C enters an insulated mixing chamber with a mass flow rate of mh=0.75 kg/s where it is mixed with a stream of cold water at 25°℃ in a steady state steady flow process. It is desired that the mixture leaves the chamber at 45°C as shown in the Figure Hot water P₁=300 kPa T₁=90°C m=0.75 kg/s Cold water P: 300 kPa T:=25C m=? 2 Mixing Chamber 3 Mixed warm water Ps=300 kPa T3=45°C m3 For Question 1: The specific enthalpy of the hot water, h1, in kJ/kg is
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question

Transcribed Image Text:D Question 1
Use this figure for Questions 1 to 5.
A hot-water stream at 90 °℃ enters an
insulated mixing chamber with a mass
flow rate of mh=0.75 kg/s where it is
mixed with a stream of cold water at
25°℃ in a steady state steady flow
process. It is desired that the mixture
leaves the chamber at 45°℃ as shown in
the Figure
Hot water
P₁=300 kPa
T₁=90°C
m=0.75 kg/s
Cold water
P-300 kPa
T: 25%
m=?
1
2
Mixing
Chamber
Mixed warm water
Ps-300 kPa
T=45°C
m3
For Question 1: The specific enthalpy of
the hot water, h1, in kJ/kg is
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY