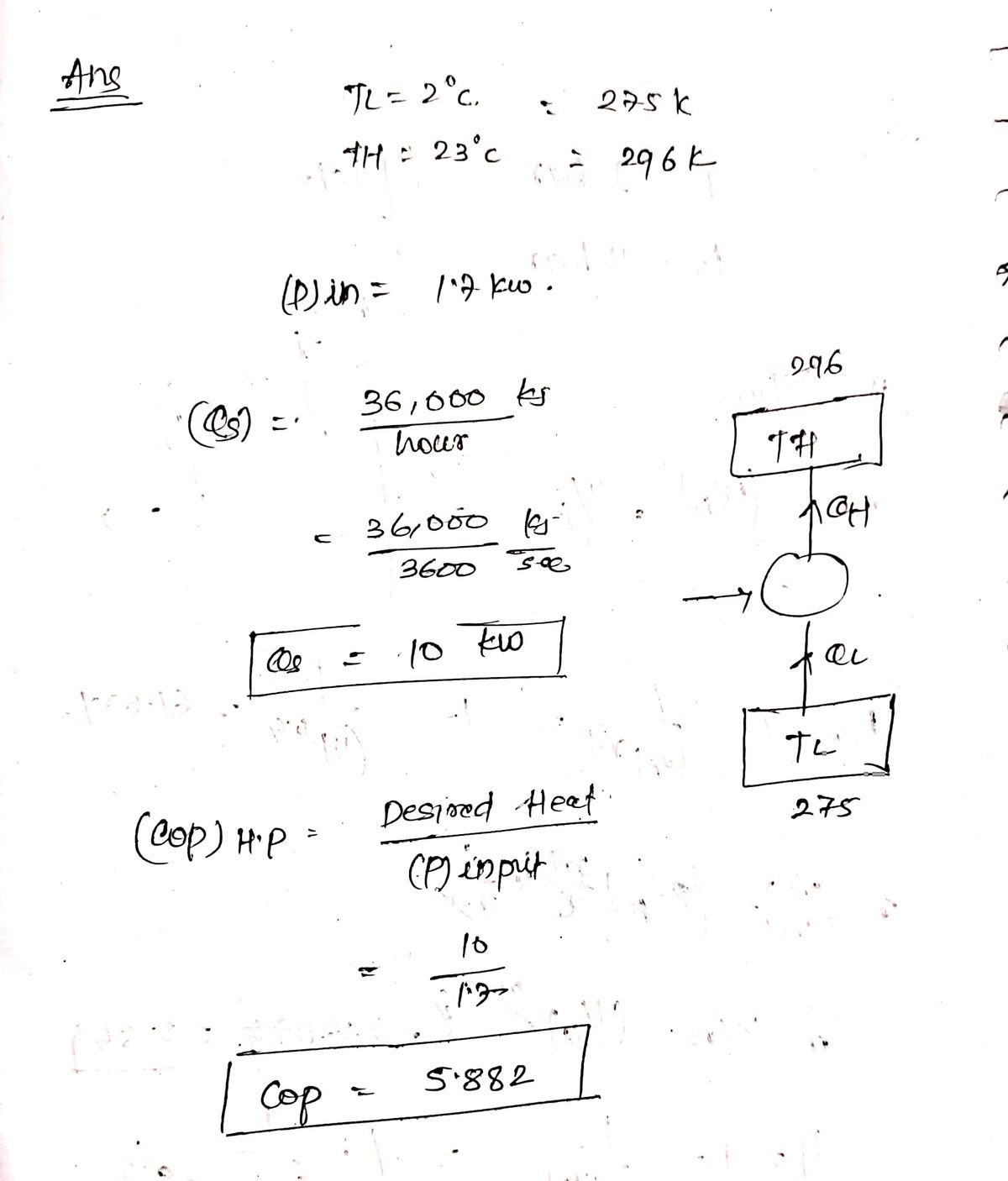
- A heat rate of 36,000 kJ/hr. a. What is the COP of the heat pump if the power consumed is 1.7 kW? b. What is the maximum COP of the heat pump? pump absorbs heat from the cold outdoors at 2° C and supplies heat to a house at 23° C at a
- A heat rate of 36,000 kJ/hr. a. What is the COP of the heat pump if the power consumed is 1.7 kW? b. What is the maximum COP of the heat pump? pump absorbs heat from the cold outdoors at 2° C and supplies heat to a house at 23° C at a
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
100%

Transcribed Image Text:**Heat Pump Performance Analysis**
A heat pump absorbs heat from the cold outdoors at 2°C and supplies heat to a house at 23°C at a rate of 36,000 kJ/hr.
a. What is the COP (Coefficient of Performance) of the heat pump if the power consumed is 1.7 kW?
b. What is the maximum COP of the heat pump?
**Note on COP**: The COP of a heat pump is a measure of its efficiency, calculated by comparing the heat output to the electrical energy input. The maximum theoretical COP can be determined using the temperatures of the heat source and sink.
**Conversion Factors**:
- Power: 1 kW = 1,000 watts
- Energy: 1 kJ = 1,000 joules
- 1 hour = 3,600 seconds
This exercise involves calculations to determine the efficiency and theoretical potential of the heat pump based on given operational temperatures and energy rates.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY