Write the net ionic equation, which should include the charges, and the phases of each species in the reaction, along with it being balanced.

Hydrochloric acid is a stronger acid than sulfurous acid and it replaces the anion when HCl reacts with the salt of sulfurous acid. The balanced chemical reaction is given below:
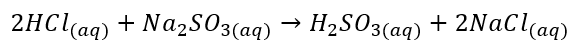
The stoichiometry of the balanced chemical reaction states that two moles of HCl react with one mole of sodium sulfate to form one mole of sulfurous acid and two moles of sodium chloride.
The ionic equation for the above reaction is given by:
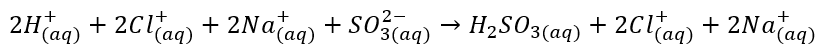
Net ionic equation: The ions who do not participate in the reaction and present in reactants and in the products as it is known as the spectator ions. In the net ionic equation, we do not write the spectator ions.
The net ionic equation for the given reaction is as follows:
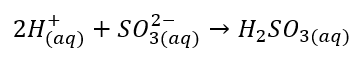
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 4 images









