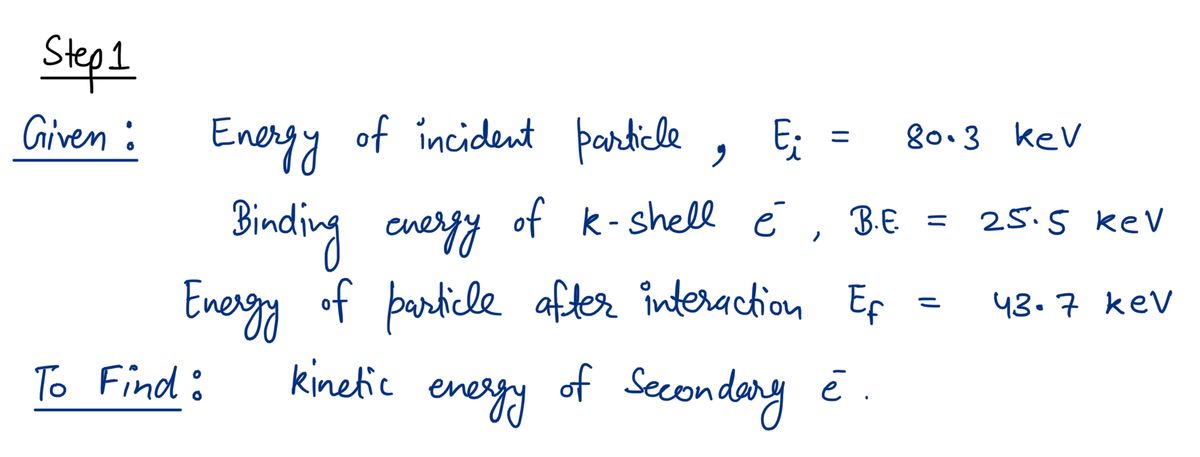
A charged particle with initial kinetic energy of 80.3 keV ionizes an electron in the K shell of a silver atom. The binding energy for K-shell electrons in silver is 25.5 keV. The charged particle has kinetic energy of 43.7 keV after the interaction. What is the kinetic energy of the secondary electron, after it is ejected from the silver atom?
A charged particle with initial kinetic energy of 80.3 keV ionizes an electron in the K shell of a silver atom. The binding energy for K-shell electrons in silver is 25.5 keV. The charged particle has kinetic energy of 43.7 keV after the interaction. What is the kinetic energy of the secondary electron, after it is ejected from the silver atom?
Related questions
Question

Transcribed Image Text:A charged particle with initial kinetic energy of 80.3 keV ionizes an electron in the K shell
of a silver atom. The binding energy for K-shell electrons in silver is 25.5 keV. The
charged particle has kinetic energy of 43.7 keV after the interaction. What is the kinetic
energy of the secondary electron, after it is ejected from the silver atom?
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images
