A cell at 25oC is described by the following cell notation Zn(s) | Zn2+(aq) || Zn2+(aq) | Zn(s) The concentration of zinc ions at the cathode is 0.50M while the concentration of zinc ions at the anode is 0.10M. Based on the given cell notation and concentrations, (show proper units and box final answers) A) Calculate the standard free energy change. B) Calculate the cell potential. C) If the given concentrations of zinc ions for anode and cathode are reversed, will the reaction proceed forward or backward?
A cell at 25oC is described by the following cell notation
Zn(s) | Zn2+(aq) || Zn2+(aq) | Zn(s)
The concentration of zinc ions at the cathode is 0.50M while the concentration of zinc ions at the anode is 0.10M. Based on the given cell notation and concentrations, (show proper units and box final answers)
A) Calculate the standard free energy change.
B) Calculate the cell potential.
C) If the given concentrations of zinc ions for anode and cathode are reversed, will the reaction proceed forward or backward?
The given cell is:
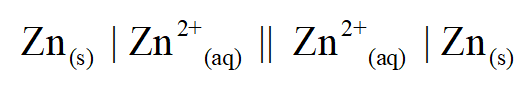
Temperature = 25˚C = 298 K
a) Since it is a concentration cell, i.e. both the cathode and anode are made up of same electrodes, thus E˚cell will be 0.
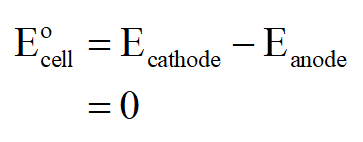
Since E˚cell is zero, hence ∆G˚ will also be zero as:
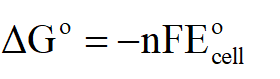
Thus standard free energy change will be 0.
Step by step
Solved in 2 steps with 5 images









