A → B + C + D being carried out isothermally in an ideal plug-flow reactor PFR) packed with a heterogeneous catalyst. The reactor operates at essentially atmospheric pressure. At the conditions of oper- ation, the ideal gas laws are obeyed. The reaction follows the rate equation -TA = KC² At the operating temperature of the reactor, k = 0.40 (mº/g mol- kg-min) The feed to the reactor is a mixture of 2 mol of A and 1 mol of steam. Neither B, C, nor D is H₂O (steam). The molar feed rate of A is 100,000 g-mol/min and the concentration of A in the feed is 10.3 g-mol/m³. Assuming that transport resistances are negligible, what
A → B + C + D being carried out isothermally in an ideal plug-flow reactor PFR) packed with a heterogeneous catalyst. The reactor operates at essentially atmospheric pressure. At the conditions of oper- ation, the ideal gas laws are obeyed. The reaction follows the rate equation -TA = KC² At the operating temperature of the reactor, k = 0.40 (mº/g mol- kg-min) The feed to the reactor is a mixture of 2 mol of A and 1 mol of steam. Neither B, C, nor D is H₂O (steam). The molar feed rate of A is 100,000 g-mol/min and the concentration of A in the feed is 10.3 g-mol/m³. Assuming that transport resistances are negligible, what
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Could it please be hand written

Transcribed Image Text:1. The cracking reaction
A → B + C + D
is being carried out isothermally in an ideal plug-flow reactor
(PFR) packed with a heterogeneous catalyst. The reactor operates
at essentially atmospheric pressure. At the conditions of oper-
ation, the ideal gas laws are obeyed. The reaction follows the rate
equation
-TA = KC²
At the operating temperature of the reactor, k = 0.40 (m/g mol-
kg-min)
The feed to the reactor is a mixture of 2 mol of A and 1 mol
of steam. Neither B, C, nor D is H₂O (steam). The molar feed rate
of A is 100,000 g-mol/min and the concentration of A in the feed
is 10.3 g-mol/m³.
Assuming that transport resistances are negligible, what
weight of catalyst is required to achieve 85% fractional con-
version of A?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 5 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
why did you use the x-1=t and x=0..., and how did you end up with 56 and 49 on the line below?
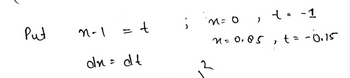
Transcribed Image Text:Put
n-1
= t
dn = dt
;
n = 0 1
n=0.05
t = -1
"
t = -0,15
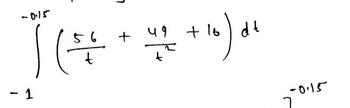
Transcribed Image Text:,
-0.15
11 + +10)에
56
t
49
k
- 1
-0.15
Solution
Follow-up Question
How did you get 1/9 and the (4x+3)^2/(x-1)^2
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The