a) Add the missing particles required to satisfy our laws of physics. p = uud, n = ddu, л¹ = ud, л¯¯ = ūd, лº = uu or dd.
a) Add the missing particles required to satisfy our laws of physics. p = uud, n = ddu, л¹ = ud, л¯¯ = ūd, лº = uu or dd.
Related questions
Question

Transcribed Image Text:**a) Add the missing particles required to satisfy our laws of physics.**
\( p = uud, \ n = ddu, \ \pi^+ = u\bar{d}, \ \pi^- = \bar{u}d, \ \pi^0 = u\bar{u} \ \text{or} \ d\bar{d}. \)
1. \( \tau^- \rightarrow e^- + \underline{\hspace{1cm}} \)
2. \( n \rightarrow p + \underline{\hspace{1cm}} \)
3. \( \pi^0 \rightarrow e^- + \nu^+_\mu + \underline{\hspace{1cm}} \)
4. \( p + n \rightarrow p + p + \bar{p} + \underline{\hspace{1cm}} \)
**b) For reactions (1) and (4) in part a), can the initial particles be at rest? Why/why not?**
Expert Solution
Step 1: The quark content of proton and neutron
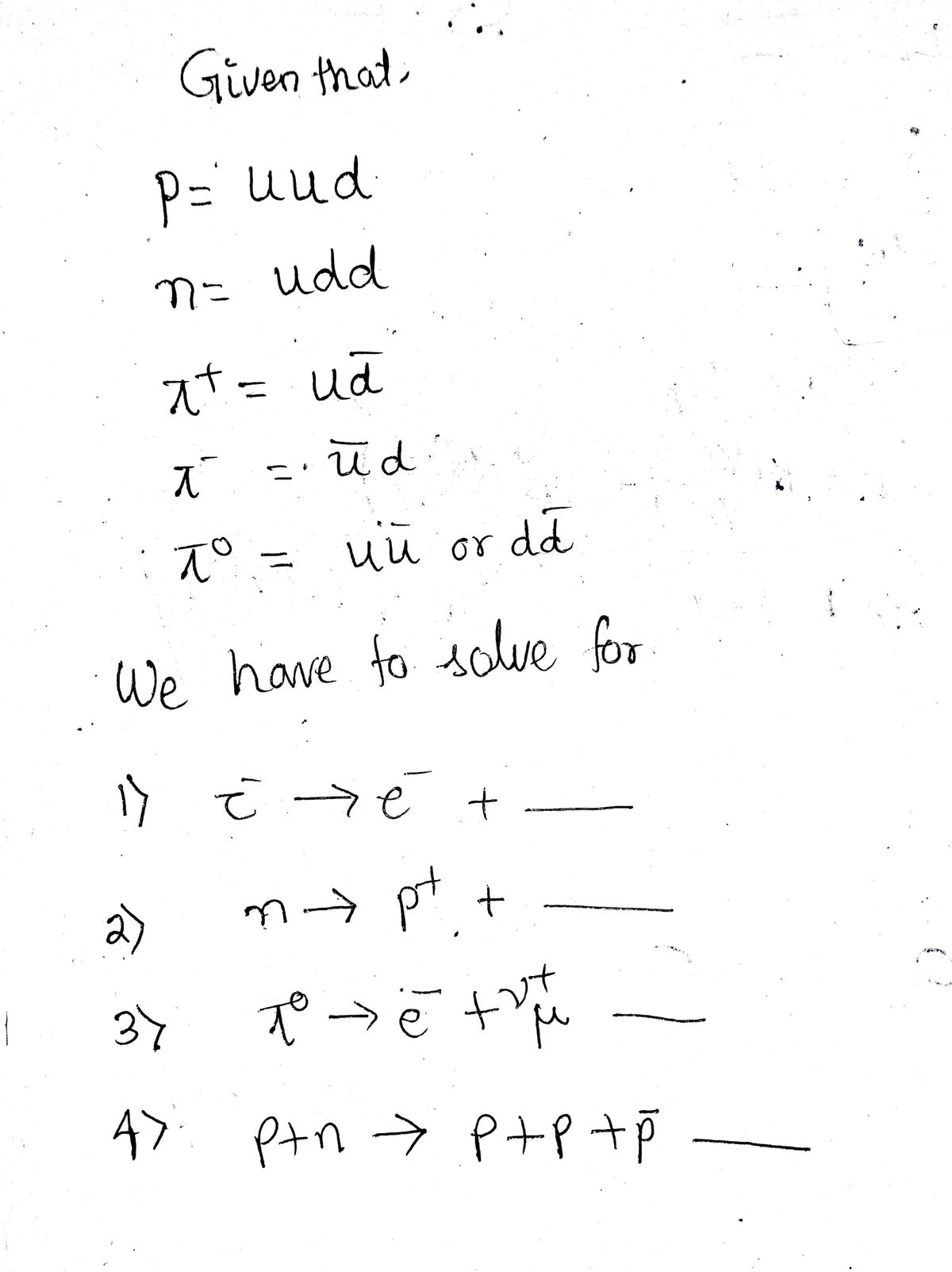
Step by step
Solved in 5 steps with 3 images
