A 500.0 mL buffer solution is 0.150M in acetic acid (Ka = 1.8 x 10-5) and 0.350M in sodium acetate. What is the pH of the solution before and after adding 0.0026 mol of NaOH? (Assume the volume of the buffer does not change)
A 500.0 mL buffer solution is 0.150M in acetic acid (Ka = 1.8 x 10-5) and 0.350M in sodium acetate. What is the pH of the solution before and after adding 0.0026 mol of NaOH? (Assume the volume of the buffer does not change)
Acetic acid is the weak acid so pH of given titrating mixture is calculated as follows,
Given,
0.150M acetic acid.
0.350M of sodium acetate
Ka of acetic acid = 1.8x10-5
Find the moles of acid and base that present in given solution.
The ICE table is,
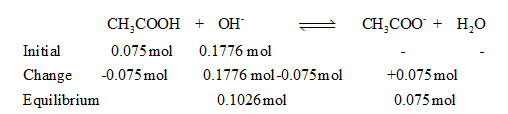
From the table pH of the solution is,
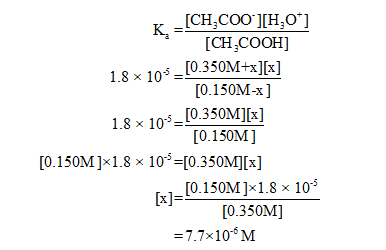
x = H3O+ ion concentration in solution
Hence, pH of the solution is,
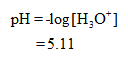
Answer: the pH of given titrating mixture is 5.11.
Step by step
Solved in 2 steps with 5 images









