A 25.0 mL sample of 0.100 M acetic acid is titrated with 0.125 M NaOH . What is the pH of the solution after 20.0 mL of NaOH have need added? (Hint: What is in solution after the addition of the NaOH?)
A 25.0 mL sample of 0.100 M acetic acid is titrated with 0.125 M NaOH . What is the pH of the solution after 20.0 mL of NaOH have need added? (Hint: What is in solution after the addition of the NaOH?)
If any base (strong base) gets added into weak acid then buffer solution formation occurs in which salt gets yielded as formed species. This solution posses specific pH depending over the volume/concentration of acid-base. This solution's alkalinity or acidity gets expressed by its "pH".
Given
The volume of acetic acid is 25.0 mL.
The concentration of acetic acid is 0.100 M.
The concentration of NaOH is 0.125 M.
The volume of NaOH is 20.0 mL.
The reaction for the formation of sodium acetate is shown below.
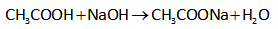
Calculation of the pH on adding 20.0 mL of NaOH is added.
The number of moles of acetic acid can be calculated as shown below.
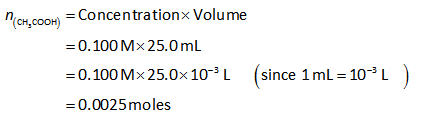
Similarly, the number of moles of NaOH is calculated can be calculated as shown below.
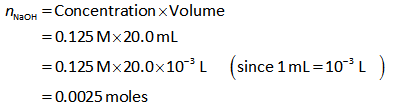
The ICE table is thus shown below.
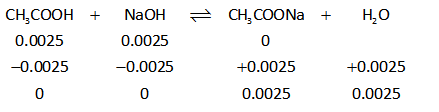
Thus the solution is formed will be buffer.
Since the number of moles of NaOH is equal to the number of moles of acetic acid. This indicates that the solution is reached at the endpoint.
At endpoint, acid, as well as base, get completely consumed in the reaction to form sodium acetate.
The total volume of solution can be calculated as shown below
(25.0+20.0) mL=45 mL.
Step by step
Solved in 6 steps with 8 images









