A 25 Celsius aqueous solution is made by adding 2.31 liters of 0.348 M NaNO2 to 3.36 liters of 0.118 HNO2. The Ka of HNO2 is 4.7x10-4. What is the pH of this resultant solution?
A 25 Celsius aqueous solution is made by adding 2.31 liters of 0.348 M NaNO2 to 3.36 liters of 0.118 HNO2. The Ka of HNO2 is 4.7x10-4. What is the pH of this resultant solution?
Interpretation: The given question is based on calculation of pH of a given buffer solution.
Concept introduction : A buffer solution is a solution that resist change in pH when small amount of an acid or base is added or when the solution is diluted.
The given solution is a mixture of weak acid NaNO2 and it's salt with strong base.
(NaNO2 salt of sodium hydroxide and nitrous acid)
To calculate pH of such buffer solution we can use Henderson-Hasselbalch equation. A general form can be written for weak and HA that inonised to ionises to it's salt and H3O+ is as follows:
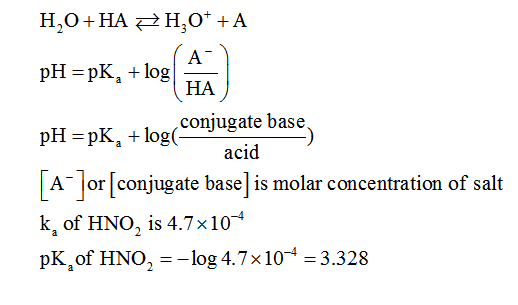
Step by step
Solved in 4 steps with 4 images









