A 10.0 L tank at 23.7 °C is filled with 5.14 g of boron trifluoride gas and 13.4 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. mole fraction: boron trifluoride partial pressure: ||atm ? mole fraction: dinitrogen difluoride partial pressure: atm Total pressure in tank: atm
A 10.0 L tank at 23.7 °C is filled with 5.14 g of boron trifluoride gas and 13.4 g of dinitrogen difluoride gas. You can assume both gases behave as ideal gases under these conditions. Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Be sure your answers have the correct number of significant digits. mole fraction: boron trifluoride partial pressure: ||atm ? mole fraction: dinitrogen difluoride partial pressure: atm Total pressure in tank: atm
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Problem Description:**
A 10.0 L tank at 23.7°C is filled with 5.14 g of boron trifluoride gas and 13.4 g of dinitrogen difluoride gas. Assume both gases behave as ideal gases under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Make sure your answers have the correct number of significant digits.
**Diagram/Chart Explanation:**
The diagram consists of a table divided into two main sections corresponding to each gas—boron trifluoride and dinitrogen difluoride—and a section for the total pressure in the tank. Each section has fields to input the mole fraction and partial pressure of the respective gas in atm. There is also a field for the total pressure in the tank.
- **Boron Trifluoride:**
- Mole fraction: [Input Box]
- Partial pressure: [Input Box] atm
- **Dinitrogen Difluoride:**
- Mole fraction: [Input Box]
- Partial pressure: [Input Box] atm
- **Total Pressure in Tank:**
- [Input Box] atm
A small tool icon with an 'x10' checkbox is displayed, presumably for notation or calculation adjustments.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F03fa78cd-6ce9-4582-bb7c-dbeac4c245c8%2F2a715672-933d-414a-8f1a-b87b58aa0389%2Fxey4hnc_processed.png&w=3840&q=75)
Transcribed Image Text:**Problem Description:**
A 10.0 L tank at 23.7°C is filled with 5.14 g of boron trifluoride gas and 13.4 g of dinitrogen difluoride gas. Assume both gases behave as ideal gases under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Make sure your answers have the correct number of significant digits.
**Diagram/Chart Explanation:**
The diagram consists of a table divided into two main sections corresponding to each gas—boron trifluoride and dinitrogen difluoride—and a section for the total pressure in the tank. Each section has fields to input the mole fraction and partial pressure of the respective gas in atm. There is also a field for the total pressure in the tank.
- **Boron Trifluoride:**
- Mole fraction: [Input Box]
- Partial pressure: [Input Box] atm
- **Dinitrogen Difluoride:**
- Mole fraction: [Input Box]
- Partial pressure: [Input Box] atm
- **Total Pressure in Tank:**
- [Input Box] atm
A small tool icon with an 'x10' checkbox is displayed, presumably for notation or calculation adjustments.
Expert Solution
Step 1
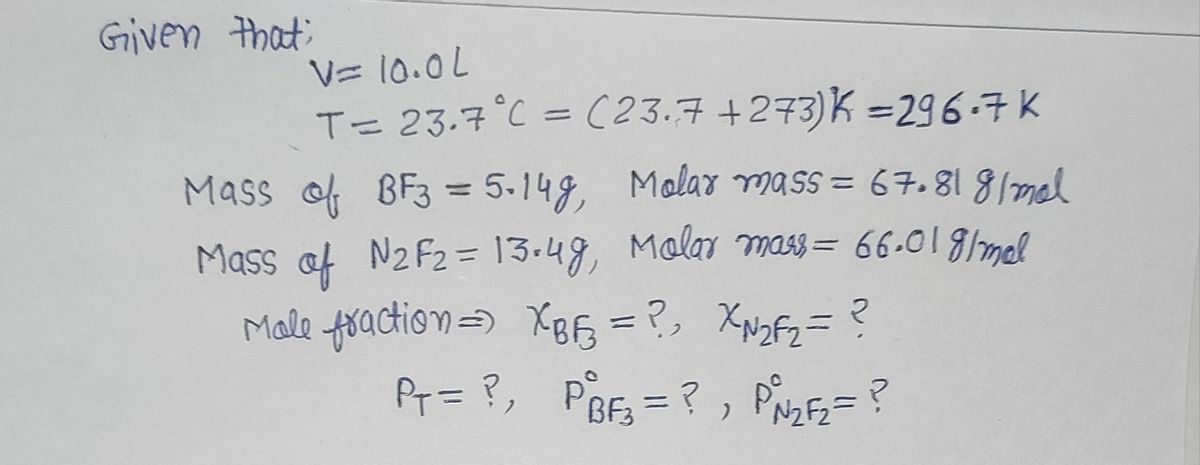
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY