A 1.00 L bottle of carbonated soda contains 5.40 g of carbon dioxide dissolved in it. If the evaporating carbon dioxide is trapped in a cylinder at 1.00 atm and 22.0°C, what volume (in L) does the gas occupy? L
A 1.00 L bottle of carbonated soda contains 5.40 g of carbon dioxide dissolved in it. If the evaporating carbon dioxide is trapped in a cylinder at 1.00 atm and 22.0°C, what volume (in L) does the gas occupy? L
Related questions
Question
Could I please get a detailed response my measurements are not exact.

Transcribed Image Text:A 1.00 L bottle of carbonated soda contains 5.40 g of carbon dioxide dissolved in it. If the evaporating carbon dioxide is trapped in a cylinder at 1.00 atm and 22.0°C, what volume (in L) does the gas
occupy?
Expert Solution
Step 1
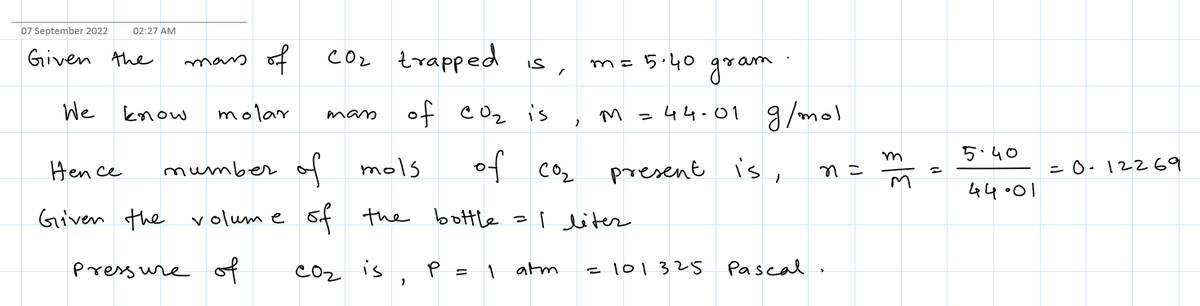
Step by step
Solved in 2 steps with 2 images
