Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
8

Transcribed Image Text:Alkyl Halides (X = CI, Br or I) :
CH3X
A
В
E
F
Alcohols
OH
HO
HO
CH3OH
AA
BB
CC
DD
ЕЕ
FF
GG
Ketones, Aldehydes and Epoxides: Assume "then H,0" is included if a protonation step is needed
H.
H.
Ph
Ph
Ph
J
K
P
Q
R
Other reagents
21 SOCI2, pyridine
22 TSCI, pyridine
23 Mg, Et20
24 MeMgBr, then H20
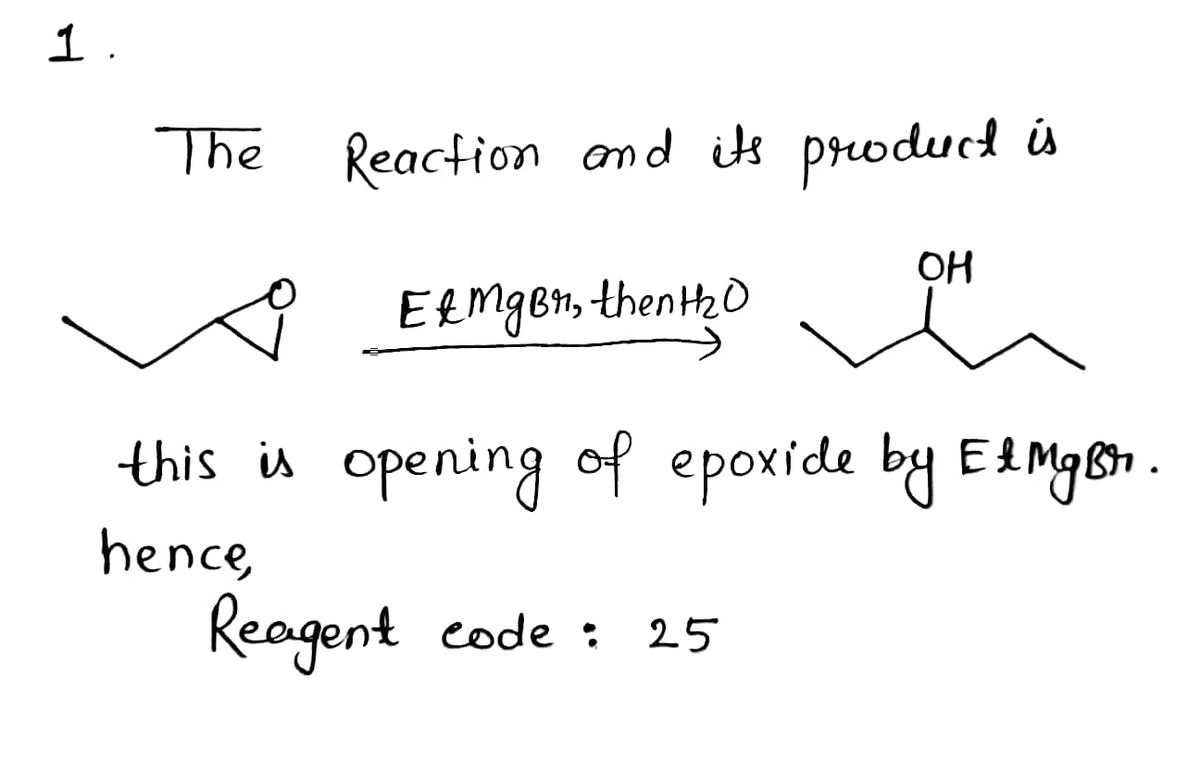
25 EtMgBr, then H20
26 PhMgBr, then H20
27 PhCH2MgBr, then H20
1 H30* (dilute H2SO4)
2 conc. H2S04, heat
11 PCC in CH2CI2
12 Na2Cr207, H2SO4, H2O
13 BH3•THF or 9-BBN, then H2O2, NaOH
14 Hg(OAc)2, H20, then NABH4
15 03, then Zn, HCI or DMS
16 МСРВА or CH;COҙH
17 Br2, light
3 NaOEt
4 t-BUOK
5 H2, Pt
6 H2, Lindlar's catalyst
7 Na, NH3
8 LAH or xs LAH, then H20
18 HBr
9 NaBH4, CH3ОН
19 HBr, ROOR
20 PBR3

Transcribed Image Text:From the reagent list, identify the reagent needed for each transformation below:
OH
Reagent code:
OH Reagent code:
OH
Reagent code:
A Moving to another question will save this response.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY