Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:8 https://openvellum.ecollege.com/course.html?courseld=16914186&OpenVellumHMAC=c662ec09ac8023bd1b971c039afd0082#10001
<Chapter 8 Problem Set
Exercise 8.93
< 5 of 20 >
I Review | Constants| Periodic Table
The quantum yield of light-induced chemical reactions (called photochemical reactions) measures the efficiency of the process. The quantum yield, , is defined
as d = number of reaction events
TUmber of photona abeorbed Suppose a quantum yield for the reaction CH3XCH3 +X is o = 0.97. A cuvette containing a solution of CH,X is irradiated with 280-nm with a power
of 885 mW for 10.0 minutes.
• Part A
Assuming total absorption of the light by the sample, what is the maximum amount (in moles) of CH3X that breaks apart?
Express your answer to two significant figures.
?
mol CH,X actual
Submit
Request Answer
Provide Feedback
Next >
81%
9:40 PM
10/19/2021
Home
End
Insert
Delete
F10
&
Backspace
NumLock
6
7
8.
Y
7
8
Home
H JK L
5
Enter
B
N M
Shift
End
PriSc
Cir
Pup
PDn
44
Expert Solution
Step 1
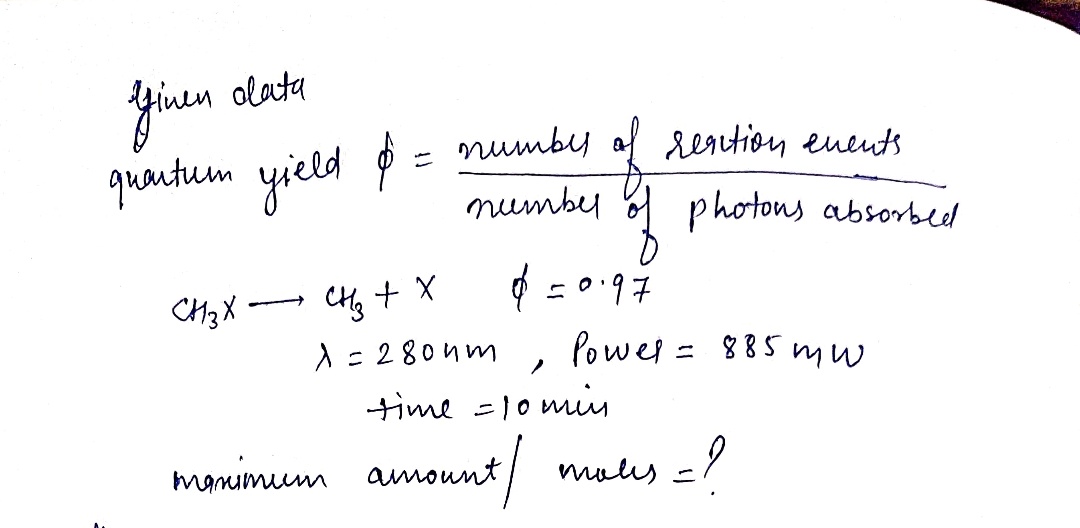
Step by step
Solved in 2 steps with 2 images

Similar questions
Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY