6. Steam enters a pressure reducing valve at a pressure of 2000 kPa, dry saturated. It is throttled adiabatically to 100 kPa. What is the temperature and internal energy of the steam at the lower pressure. (162 °C, 2600 kJ/kg)
6. Steam enters a pressure reducing valve at a pressure of 2000 kPa, dry saturated. It is throttled adiabatically to 100 kPa. What is the temperature and internal energy of the steam at the lower pressure. (162 °C, 2600 kJ/kg)
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Cn yu do question 6
![1. A boiler feedpump receives 10 °C water at a pressure of 500 kPa. The discharge
pressure is 2 400 kPa. Given a pump capacity of 160 000 kg/h, and after correcting.
for the temperature of the water, what is the power supplied to the water? (8 kW)
2. Water is supplied to a pump at a pressure of 200 kPa. The power transferred to the
water is estimated to be 30 kW. The pump discharge pressure is 2 200 kPa and the
specific volume is 0.00106 m3/kg. Estimate the amount of water pumped in cubic
meters per min. (0.90 m3/min.)
3. Air enters a compressor with a velocity of 6.0 m/s and an enthalpy of 233 kJ/kg and
leaves with a velocity of 12 m/s and an enthalpy of 477 kJ/kg. Assuming adiabatic
compression and negligible change in potential energy, calculate the work required to
compress 1.0 kg of air. (244 kJ/kg)
4. An air compressor is supplied with air at 101.3 kPa and a specific volume of 0.804
m3/kg. The air is discharged at 700 kPa and a specific volume of 0.162 m³/kg. The
initial and final internal energies are 37.2 kJ/kg and 114 kJ/kg respectively. The
cooling water removes 88.0 kJ/kg of air compressed. The change in kinetic and
potential energy may be neglected. Calculate (a) the specific work required, (b) the
power required given a mass flow rate of 45.6 kg/min. Also (c) if the heat being
rejected is transferred to cooling water flowing through a coil, and the cooling water
picks up 10ºC across that coil, and the pump forcing that cooling flow has a 100 kPa
AP across it, calculate the power required to drive the cooling water pump (197 kJ/kg.
150 kW, 160 W)
5. Wet steam is throttled from 1000 kPa to 10 kPa and 130 °C. Find the dryness fraction
at state 1. [98.3%]
6. Steam enters a pressure reducing valve at a pressure of 2000 kPa, dry saturated. It is
throttled adiabatically to 100 kPa. What is the temperature and internal energy of the
steam at the lower pressure. (162 °C, 2600 kJ/kg)
7. A pressure reducing valve receives 5 kg/s of steam at 2MPa with a specific volume of
0.1255 m³/kg and an internal energy of 2774 kJ/kg. If the pressure downstream of
the throttle is 200 kPa and the velocity at that point is 4.25 m/s, determine to the
nearest inch the required ID of the downstream piping in order to accommodate the
volume flow rate. [54.0 inches]
DVI & Dual HDMI inputs for high-definition support
ho](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8f9da62a-a094-47dc-9747-5a0eb30c4375%2F853f5bb7-3b02-4367-a1b2-6142ce6b5eff%2Fztopthu_processed.jpeg&w=3840&q=75)
Transcribed Image Text:1. A boiler feedpump receives 10 °C water at a pressure of 500 kPa. The discharge
pressure is 2 400 kPa. Given a pump capacity of 160 000 kg/h, and after correcting.
for the temperature of the water, what is the power supplied to the water? (8 kW)
2. Water is supplied to a pump at a pressure of 200 kPa. The power transferred to the
water is estimated to be 30 kW. The pump discharge pressure is 2 200 kPa and the
specific volume is 0.00106 m3/kg. Estimate the amount of water pumped in cubic
meters per min. (0.90 m3/min.)
3. Air enters a compressor with a velocity of 6.0 m/s and an enthalpy of 233 kJ/kg and
leaves with a velocity of 12 m/s and an enthalpy of 477 kJ/kg. Assuming adiabatic
compression and negligible change in potential energy, calculate the work required to
compress 1.0 kg of air. (244 kJ/kg)
4. An air compressor is supplied with air at 101.3 kPa and a specific volume of 0.804
m3/kg. The air is discharged at 700 kPa and a specific volume of 0.162 m³/kg. The
initial and final internal energies are 37.2 kJ/kg and 114 kJ/kg respectively. The
cooling water removes 88.0 kJ/kg of air compressed. The change in kinetic and
potential energy may be neglected. Calculate (a) the specific work required, (b) the
power required given a mass flow rate of 45.6 kg/min. Also (c) if the heat being
rejected is transferred to cooling water flowing through a coil, and the cooling water
picks up 10ºC across that coil, and the pump forcing that cooling flow has a 100 kPa
AP across it, calculate the power required to drive the cooling water pump (197 kJ/kg.
150 kW, 160 W)
5. Wet steam is throttled from 1000 kPa to 10 kPa and 130 °C. Find the dryness fraction
at state 1. [98.3%]
6. Steam enters a pressure reducing valve at a pressure of 2000 kPa, dry saturated. It is
throttled adiabatically to 100 kPa. What is the temperature and internal energy of the
steam at the lower pressure. (162 °C, 2600 kJ/kg)
7. A pressure reducing valve receives 5 kg/s of steam at 2MPa with a specific volume of
0.1255 m³/kg and an internal energy of 2774 kJ/kg. If the pressure downstream of
the throttle is 200 kPa and the velocity at that point is 4.25 m/s, determine to the
nearest inch the required ID of the downstream piping in order to accommodate the
volume flow rate. [54.0 inches]
DVI & Dual HDMI inputs for high-definition support
ho
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Can you please use these steam tables
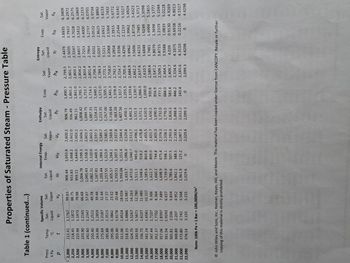
Transcribed Image Text:Table 1 (continued...)
Press
k Pa
р
Properties of Saturated Steam - Pressure Table
Temp
°℃
t
Specific Volume
Sat.
Liquid
Vf
Sat.
Vapor
Vg
Note: 100k Pa = 1 Bar = 100,000N/m²
Sat.
Liquid
Uf
Internal Energy
Evap.
All
Sat.
Vapor
Ug
Enthalpy
Sat.
Liquid
hf
Evap.
Sat.
Vapor
hg
Entropy
Sat.
Liquid
Sf
Evap.
Ufg
hfg
Sfg
2,000
2,799.5
3.8935 6.3409
1,890.7
2.4474
2,799.5
2.5035
3.7937
6.2972
1,865.2
2,250
212.42 1.1767
218.45 1.1872
223.99 1.1973
233.90 1.2165
3.7028 6.2575
3.5412 6.1869
2,500
3,000
3,500
99.63
88.75
79.98
66.68
57.07
49.78
39.44
242.60 1.2347
3.4000 6.1253
3.2737 6.0701
4,000
250.40 1.2522
3.2737
3.0532 5.9734
5,000
263.99 1.2859
39.44
275.64 1.3187 32.44
2.8625 5.8892
2.6922 5.8133
285.88 1.3513
295.06 1.3842
303.40 1.4178
1,316.64
1,316.64
906.44 1,693.8 2,600.3 908.79
936.49
933.83 1,668.2 2,602.01
1,865.2 2,801.7
1,841.0 2,803.1 2.5547
959.11 1,644.0 2,603.1 962.11
1,008.42 1,795.7 2,804.2 2.6457
1,004.78 1,599.3 2,604.1
1,045.43 1,558.3 2,603.7 1,049.75 1,753.7 2,803.4
2.7253
2.7964
1,082.31 1,520.0 2,602.3 1,087.31 1,714.1 2,801.4
1,147.81 1,449.3 2,597.1 1,154.23 1,640.1 2,794.3 2.9202
1,213.35 1,571.0 2,784.3 3.0267
6,000
1,205.44 1,384.3 2,589.7
1,267.00 1,505.1 2,772.1 3.1211
7,000
27.37 1,257.55 1,323.0 2,580.5
1,441.3 2,758.0 3.2068 2.5364 5.7432
1,441.3
8,000
1,305.57 1,264.2 2,569.8
23.52
1,350.51 1,207.3 2,557.8
1,378.9 2,742.1 3.2858 2.3915 5.6772
9,000
20.48
3.3596 2.2544 5.6141
1,393.04 1,151.4 2,544.4
1,317.1 2,724.7
10,000 311.06 1.4524 18.026
3.4295 2.1233 5.5527
1,433.7 1,096.0 2,529.8
11,000 318.15 1.4886 15.987
3.4962 1.9962 5.4924
1,473.0 1,040.7 2,513.7
12,000 324.75 1.5267 14.263
13,000 330.93 1.5671 12.780 1,511.1
2,496.1 1,531.5
985.0
14,000 336.75 1.6107 11.485 1,548.6 928.2 2,476.8
15,000 342.24 1.6581 10.337 1,585.6 869.8 2,455.5
16,000 347.44 1.7107 9.306 1,622.7 809.0 2,431.7
17,000 352.37 1.7702
744.8
1,255.5
2,705.6
1,363.26
1,407.56
1,450.1
1,491.3
1,531.5
1,571.1
1,193.6
2,684.9
1,130.7
2,662.2
3.5606
1.8718
5.4323
1,066.5
2,637.6
1.7485 5.3717
1,610.5
1,000.0
2,610.5
1,650.1
2,580.6
8.364
1,660.2
2,405.0
1,690.3
1,698.9
675.4
2,374.3
1,732.0
18,000 357.06 1.8397 7.489
19,000 361.54
930.6
856.9
777.1
688.0
583.4
3.6232
3.6848 1.6249 5.3098
3.7461 1.4994 5.2455
3.8079 1.3698 5.1777
2,547.2
3.8715 1.2329 5.1044
2,509.1 3.8715
3.9388 1.0839 5.0228
2,464.5
0.9130 4.9269
4.039
2,409.7
4.1075
2,334.6
0.6938 4.8013
2,165.6 4.3110
2,099.3 4.4298
1.9243
6.657
598.1
2,338.1
1,776.5
5.834
2,293.0
20,000 365.81
21,000 369.89
2.036
2.207
1,826.3
4.952
1,739.9
1,785.6 507.5
1,842.1 388.5
125.2
1,961.9
0
1,888.4
446.2
22,000 373.80 2.742
3.568
2,230.6
2,087.1 2,022.2
2,029.6
143.4
22,090
374.14
3.155
3.155
2,029.6
0.2216 4.5327
4.4298
2,099.3
0
0
O John Wiley and Sons, Inc., Keenan, Keyes, Hill, and Moore. This material has been copied under license from CANCOPY. Resale or further
copying of this material is strictly prohibited.
Sat.
Vapor
Sg

Transcribed Image Text:Table 3
Abs Press.
kPa
(Sat. Temp) 100
150
200
V v 17,196 19,512 21,825
h 2,687.5 2,783.0 2,879.5
S 8.4479 8.6882 8.9038
10
(45.81)
30
(69.10)
50
(81.33)
100
(99.63)
200
(120.23)
500
(151.86)
v
V
V 5,715 6,493
h 2,685.0
S 7.9357
h
s
V
h
300
(133.55) S
V
h
S
5,000
(263.99)
V
h
S
V
1,000 h
(179.91)
S
V
2,000 h
(212.43)
S
V
3,000 h
(233.90)
S
V
4,000 h
(250.40)
S
v
1,695.8 1,936.4 2,172 2,406
h 2,676.2 2,776.4 2,875.3 2,974.3
7.3614 7.6134 7.8343 8.0333
s
Table of Properties for Superheated Steam
V
h
S
7,267
2,781.5 2,878.6
8.3952
8.1785
V
6,000 h
(275.64) S
3,889 4,356
3,418
2,682.5
2,780.1
2,877.7
7.6947 7.9401 8.1580
Temperature Degrees Celsius
250
24,136
2,977.3
9.1002
8,040
2,976.7
8.5923
4,820
2,976.0
8.3556
***
796.4
633.9 716.3
2761.0 2,865.6 2,967.6
7.0778 7.3115 7.5166
Note: 100k Pa = 1 Bar = 100,000N/m²
232.7
206.0
2,827.9 2,942.6
6.6940 6.9247
600
700
500
400
300
26,445 31,063 35,679 40,295 44,911
3,928.7
3,076.5 3,279.6 3,489.1 3,705.4
10.4028
9.6077 9.8978 10.1608
9.2813
959.6 1,080.3 1,198.8 1,316.2
2,768.8 2,870.5 2,971.0 3,071.8
7.2795 7.5066 7.7086 7.8926
7.8926
8,811
3,076.0
8.7736
5,284
3,075.5
8.5373
2,639
3,074.3
8.2158
424.9 474.4 522.6
2,855.4 2,960.7 3,064.2
7.0592
7.2709
7.2709 7.4599
10,351
3,279.2
9.1003
111.44 125.47
2,902.5 3,023.5
6.5453 6.7664
6,209
3,278.9
8.8642
70.58 81.14
2,855.8 2,993.5
6.2872 6.5390
3,103
3,278.2
8.5435
875.3 1,031.5 1,186.7
3,069.3 3,275.0
7.7022 8.0330
3,486.0
8.3251
257.9 306.6
3,263.9
3,051.2
7.1229
7.4651
617.3
3,271.9
7.7938
1,1891
3,488.9
9.3906
99.36
3,230.9
6.9212
58.84
73.41
2,960.7 3,213.6
6.3615
6.7690
7,134
7,134 8,057
8,057
3,488.7
3,705.1
9.1546
9.4178
3,565
4,028
3,488.1
3,704.7
8.8342 9.0976
1,549.3 1,781.4 2,013 2,244
3,276.6 3,487.1 3,704.0
8.2218 8.5133 8.7770
3,927.6
9.0194
36.16
6.0674
2,884.2 3,177.2
710.9
3,483.9
8.0873
13,430
3,705.3
9.6537
354.1
3,478.5
7.7622
151.20 175.68
199.60
3,247.6 3,467.6 3,690.1
3,247.6
7.1271 7.4317
7.7024
86.43
3,445.3
7.0901
45.32 57.81
68.57
2,924.5 3,195.7 3,433.8
6.2084 6.6459
6.9759
1,341.4
3,703.2
8.5892
804.1
3,701.7
8.3522
401.1
3,697.9
8.0290
116.19 132.43
3,456.5
3,682.3
7.2338
7.5085
98.95
3,674.4
3,674.4
7.3688
78.69
3,666.5
7.2589
65.25
47.39 56.65
3,422.2
6.5408 6.8803 7.1677
3,658.4
14,969
3,928.6
19,585
16,508 18,047
4,158.9 4,396.4 4,640.5
9.8957 10.1210 10.3325 10.5322
8,981 9,904 10,828 11,751
3,928.5 4,158.9 4,396.3 4,640.5
9.6599 9.8852 10.0967 10.2964
9.6599
4,490
3,928.2
9.3398
1,495.7
3,927.1
8.8319
896.9
3,925.9
8.5952
447.8
3,923.1
8.2731
223.2
3,917.4
7.9487
800
900
1,000
49,526 54,141 58,757
4159.0 4,396.4 4,640.6
10.6281 10.8396 11.0393
110.95
3,905.9
7.6198
88.49
3,900.1
7.5122
4,952 5,414 5,875
4,158.6
4,396.1
4,640.3
9.5652
9.7767
9.9764
2,475
4,158.2
9.2449
1,649.9 1,804.1
1,958.1
4,157.8 4,395.4 4,639.7
9.0576
9.2692
9.2692 9.4690
989.6 1,082.2 1,174.7
4,156.9
4,394.7
4,639.1
8.8211
9.0329
9.2328
2,706
2,937
4,395.8
4,395.8 4,640.0
9.4566
9.6563
246.7
4,150.3
8.1765
494.3
4,154.7
8.4966 8.7118
148.38
3,911.7
7.7571 7.9862 8.1999 8.4009
164.14 197.80 195.41
4,145.9 4,385.9 4,631.6
122.87
4,141.5
7.8502
540.7 587.1
4,392.9 4,637.6
8.7118 8.9119
98.11
4,137.1
7.7440
270.0
293.3
4,389.4 4,634.6
8.3895 8.5901
134.69 146.45
4,382.3 4,628.7
8.0647 8.2662
107.62 117.07
4,378.8 4,625.7
7.9593 8.1612
73.52
89.58 97.49
81.60
4,132.7 4,375.3 4,622.7
3,894.2
7.4234 7.6566 7.8727 8.0751
Solution
Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY