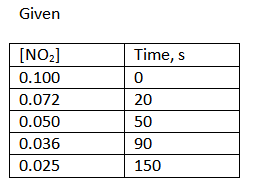
43-46 Consider the time and concentration data for: 2 NO2(g) 2 NO(g) + O2(g). Time, s [NO2] 43 a. How long is the first half-life? Write the value and units 0.100 0.072 20 b. How long is the second half-life? Write the value and units 0.050 50 44. Write the rate law for this reaction. Explain why you made this choice. 0.036 90 0.025 150 45. What is the value of the rate constant? Write the general equation, the calculation equation with units, and the answer with units 46. How much time is needed until only 0.010 M NO2 remains? Write the general equation, the calculation with units, and the answer with units c
![43-46
Consider the time and concentration data for: 2 NO2(g) 2 NO(g) + O2(g).
Time, s
[NO2]
43 a.
How long is the first half-life? Write the value and units
0.100
0.072
20
b. How long is the second half-life? Write the value and units
0.050
50
44. Write the rate law for this reaction. Explain why you made this choice.
0.036
90
0.025
150
45. What is the value of the rate constant? Write the general equation, the calculation
equation with units, and the answer with units
46. How much time is needed until only 0.010 M NO2 remains? Write the general equation, the calculation with units,
and the answer with units c](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F83ec917b-3e82-4dc1-b5e7-24fa4bff0896%2Ff946f77f-9e4c-4499-9cf7-3e5077669a2e%2Fvm3n7ml.jpeg&w=3840&q=75)
“Since you have asked multiple questions, we will solve the first question for you. If you want any specific question to be solved then please specify the question number or post only that question.”
The "half-life" basically defines the time essential for reactant's concentration to remain 50 % (half) of its actual concentration. The "second-half life" defines the time essential for reactant's concentration to remain 25 % (1/4) of its actual concentration.
Part (a):
The given reaction is as follows,
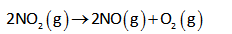
The concentration at time 0 is basically the reactant’s initial concentration.
In the given reaction the initial concentration (at time 0 s) of the reactant NO2 is 0.100 M.
Since first life is basically the time where initial concentration remains 1/2 (50%) of its original amount.

Since it is given, at 50 seconds the concentration of NO2 reaches 0.050 M (exact half of its initial concentration).
Thus the first half-life is 50 sec.
Step by step
Solved in 4 steps with 4 images









