4.00 Litres of hydraulic oil is placed in a cylinder with a movable piston, on which a heavy block is placed. The hydraulic oil pressure is maintained at 2.45x105 Pa and the temperature of the oil is raised by 45K. The oil is in the liquid phase and expands by a small amount 1.2x10-3m3. Calculate the work done on the oil and the change in internal energy of the oil. The specific heat capacity of hydraulic oil is 2300J/(kg.K) and its density is 800kg/m3
Q: F d T
A:
Q: A gas is compressed at a constant pressure of 0.293 atm from a volume of 7.62 L to 3.32 L. In the…
A: The given values are, P=0.293 atm=0.293 atm1.01325×105 Pa1 atm=29688.225 PaV1=7.62 L=7.62×10-3…
Q: An ideal gas is initially at a temperature of 300 K with a pressure of 2.5 kPa and a volume of 1 m³.…
A: Final temperature of the gas is T2 = 900 K Change in internal energy of the gas is U = 7.5 kJ
Q: To stop a volcano from destroying a local town you come up with a plan to drop a huge ice cube into…
A:
Q: A balloon is filled with 0.0020 m^3 of air at room temperature and at 1.01 x 10^5 Pa of atmospheric…
A: 1). Isothermal process is when temperature remain constant. In this process as there is no change in…
Q: A container holds 4.0 x 1022 molecules of an ideal gas at 0 °C. A piston compresses the gas, doing…
A:
Q: How much work is done on the gas in the process as shown, in Joules? Vf = 94 cm3. (1.00 cm3 =…
A: Given: Vf=94 cm31 cm3=1×10-6 m31 kPa=1×103 Pa
Q: One mole of an ideal gas first undergoes an isothermal expansion at a temperature T1 = 650 K to 5…
A:
Q: . An ideal gas is under an initial pressure 2.45 x 104 Pa and occupies a volume of 0.20 m³. The slow…
A: work done is isobaric process =Pvf-vi
Q: Three moles of an ideal monatomic gas are at a temperature of 322 K. Then 2623 J of heat is added to…
A: the first law of thermodynamics, Q=du+W2623 J=du+-745 Jdu=3368 J the change in internal energy for…
Q: Gas in a container is at a pressure of 1.8 atm and a volume of 6.0 m³. (a) What is the work done on…
A: Wordk done on the gas = -work done by the gas =-PVfinal-Vinitial=PVinitial-Vfinal
Q: (a) An ideal gas maintained at a constant pressure of 139 kPa receives 6.0x102 J of heat. If the…
A:
Q: Calculate the work done by the gas. If the internal energy of the gas decreases by 8.00 J, find the…
A:
Q: A gas expands by 1.2 L at a constant pressure of 2.5x105 Pa. During expansion 230 J of heat is…
A:
Q: A gasoline engine takes in 1.61×104 J of heat and delivers 3700 J of work per cycle. The heat is…
A:
Q: expands, it does 280 J of work. What is the change in the internal energy AU of the gas during this…
A: For an ideal gas, internal energy is only the function of temperature. And given that we have…
Q: A gas is compressed inside a cylinder. An average force of 68 N acts to move the piston 1.5 m.…
A:
Q: The cylinder's volume is initially 6.00 L, when a force on the piston of F = 19.0 kN pushes the…
A:
Q: The internal energy of a system is initially 24 J. The system does 25 J of work. What is the…
A: Given data: Initial internal energy (Ui) = 24 J Work done by the system (W) = 25 J Heat added to…
Q: One mole of an ideal gas does 1700 J of work as it expands isothermally to a final pressure of 1.00…
A: One mole of an ideal gas does 1700 J of work as it expands isothermally to a final pressure of 1.00…
Q: An ideal gas expands at a constant total pressure of 2.5 atm from 480 mL to 830 mL . Heat then flows…
A:
Q: You are boiling water in a pot on the stove for your tea. Being the brilliant scientist you are, you…
A: Given. The 0.1 kg of water in a pot is boiling at 100 degrees celsius. In the process of boiling the…
Q: A system gains 3080 J of heat at a constant pressure of 1.28 × 105 Pa, and its internal energy…
A:
Q: The initial temperature of 13 moles of an ideal gas is 358 K. 18000 J of heat are added to the gas…
A: It is given that on adding heat energy, gas gets expanded. It means that volume of gas gets…
Q: A system gains 3310 J of heat at a constant pressure of 1.38 × 105 Pa, and its internal energy…
A: Write the given values with suitable variables.
Q: A cylinder contains 0.125 mol of an ideal gas. The cylinder has a movable piston on top, which is…
A: Given:- n = 0.125 mol T1 = 25oC = 298 K T2 = 320oC = 593 K
Q: A chemical reaction transfers 8450 J of thermal energy into 11.8 moles of an ideal gas while the…
A: Given:Thermal energy,Q=8450 JNumber of moles,n=11.8Constant pressure,P=1.69 ✕ 105 PaVolume…
Q: An ideal gas is at an initial pressure of 3000 kPa and a volume of 7.00 L. The gas is expanded at…
A: Given that the value of the eshal pressure and initial volume and final volume. Then We have to…
Q: Sections 14.2 and 14.3 provide useful information for this problem. When a monatomic ideal gas…
A: Write the expression for the specific heat at constant pressure. cp=52R
Q: A gas in a cylinder expands from a volume of 0.110 m³ to 0.320 m³. Heat flows into the gas just…
A: Given data: Initial volume of gas is 0.110 m3, final volume of gas is 0.320 m3, pressure of gas is…
Q: The initial pressure and volume are 80 kPa and 0.35 m3, respectively. The final pressure and volume…
A: Given initial pressure (P1)=80 kPa initial volume (V1)=0.35 m3 final pressure (P2)=170 kPa final…
Q: A gas in a cylinder expands from a volume of 0.110 m² to 0.320 m³. Heat flows into the gas just…
A: Given data: Initial volume of gas is 0.110 m3, final volume of gas is 0.320 m3, pressure of gas is…
Q: A system with a piston expands when it gives off 51.9J of heat to the surroundings. The piston is…
A:
Q: Three moles of an ideal monatomic gas are at a temperature of 383 K. Then 2892 J of heat is added to…
A: Apply the thermodynamics first law and substitute the corresponding values to calculate the change…
Q: P₁ P3 Р 1 T 2 T₂ 3 T₁ V A heat engine consists of a heat source that causes a monatomic gas to…
A: P1=300 kPaV1=150 cm3T1=20°CV2=450 cm3CP=5R2R=8.314 J/mol.K
Q: 158 J of energy is transferred to a system consisting of 2.0 moles of an ideal gas. If the volume of…
A: Given: The amount of energy transferred is Q=158 J. The number of moles is m=2 mole. The volume is…
Q: A gas is compressed inside a cylinder. An average force of 67 N acts to move the piston 4.4 m.…
A:
4.00 Litres of hydraulic oil is placed in a cylinder with a movable piston, on which a heavy block is placed. The hydraulic oil pressure is maintained at 2.45x105 Pa and the temperature of the oil is raised by 45K. The oil is in the liquid phase and expands by a small amount 1.2x10-3m3. Calculate the work done on the oil and the change in internal energy of the oil. The specific heat capacity of hydraulic oil is 2300J/(kg.K) and its density is 800kg/m3
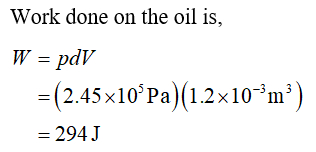
Step by step
Solved in 2 steps with 2 images

- 10. A container with a movable piston holds 2.00 moles of a monatomic ideal gas at a pressure of 3.0 × 105 N/m2 in a volume of 0.018 m3 . (a) What is the temperature of the gas? (b) The gas undergoes an isothermal expansion to a volume of 0.027 m3 . How much work does the gas do during this expansion? (c) How much heat flows into or out of the gas during this expansion? Does it flow into or out of the gas?The water in a deep underground well is used as the cold resevoir of a Carnot heat pump that maintains the temperature of a house at 303 K. To deposit 13000 J of heat in the house, the heat pump requires 716 J of work. Determine the temperature of the well water.A gas is enclosed in a container fitted with a piston of cross-sectional area 0.150 m2. The pressure of the gas is maintained at 7,200 Pa as the piston moves inward 23.5 cm. (a) Calculate the work done by the gas. J(b) If the internal energy of the gas decreases by 7.50 J, find the amount of energy removed from the system by heat during the compression. J
- An ideal monatomic gas is contained in a vessel of constant volume 0.380 m3. The initial temperature and pressure of the gas are 300 K and 5.00 atm, respectively. The goal of this problem is to find the temperature and pressure of the gas after 19.0 kJ of thermal energy is supplied to the gas. (b) Find the specific heat of the gas. __________________ J/K (d) Use the first law of thermodynamics to find the change in internal energy of the gas.__________________ kJ(e) Find the change in temperature of the gas. __________________ KA steam boiler has a total volume of 4 m3. The boiler initially contains 3 m3 of liquid water and 1 m3 of equilibrium steam at 0.1 MPa. The boiler is turned on and heat is transmitted to the water and steam. Meanwhile, the inlet and outlet valves of the boiler remain closed. The safety valve escapes when the pressure reaches 5 MPa. How much heat is transmitted to the water and steam before I open the safety valve?The first law of thermodynamics relates the heat transfer into or out of a system to the change of internal and the work done on the system. How much heat, in joules, is transferred into a system when its internal energy decreases by 165 J while it was performing 27.5 J of work?
- 64870 J of heat is added to a gas at a constant pressure of 1.75 x 105 Pa This causes a change in internal energy of 2570 J. If the starting volume is .864 m3, what is the final volume?An air-filled cylinder with a movable piston has a heat reservoir in contact with it. The heat reservoir transfers 850 J of energy into the cylinder. Due to the energy transfer, the piston does 437 J of work on the environment. What is the change in internal energy of the system?Answers (~1.9kJ, ~1.1kJ, ~4kJ) The figure to the right shows a gas contained in a vertical piston-cylinder assembly. A vertical shaft with a cross- sectional area of 1.0 cm² is attached to the top of the piston. The total mass of the piston and shaft is 35 kg. While the gas is slowly heated, the internal energy of the gas increases by 0.25 kJ, the potential energy of the piston-shaft combination increases by 0.5 kJ, and a force of 1300 N is exerted on the shaft as shown in the figure. The piston and cylinder are both good insulators and friction between them is negligible. The local atmospheric pressure is 1 bar and g = 9.81 m/s². Determine: 21 F-1300N (c) the heat transfer to the gas (kJ) (d) Provide a detailed accounting of where all of the heat energy has gone. C 19 4 A-14cm (a) the work done by the shaft (kJ) (b) the work done in displacing the atmosphere (kJ) (atmosphere does not act on shaft aka a donut area) D²10.0