(4). Suppose we take n moles of a monatomic ideal gas through the following reversible cycle: AB is an isobaric compression; BC is an adiabatic expansion; CD is an isothermal expansion; DA is a constant volume pressure increase completing the cycle. (a) Show the cycle on a PV-diagram. (b) Express the heat flow AQ work W change in internal energy the change in entropy AS , for the legs AB, BC, and CD in terms of number of "int To moles n, universal ideal gas constant R. 'A 'B and
(4). Suppose we take n moles of a monatomic ideal gas through the following reversible cycle: AB is an isobaric compression; BC is an adiabatic expansion; CD is an isothermal expansion; DA is a constant volume pressure increase completing the cycle. (a) Show the cycle on a PV-diagram. (b) Express the heat flow AQ work W change in internal energy the change in entropy AS , for the legs AB, BC, and CD in terms of number of "int To moles n, universal ideal gas constant R. 'A 'B and
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
Help

Transcribed Image Text:(4). Suppose we take n moles of a monatomic ideal gas through the following reversible cycle: AB is an isobaric
compression; BC is an adiabatic expansion; CD is an isothermal expansion; DA is a constant volume pressure
ΔQ
increase completing the cycle. (a) Show the cycle on a PV-diagram. (b) Express the heat flow
, work W
change in internal energy
ДЕ
the change in entropy
Fint ,
AS
for the legs AB, BC, and CD in terms of number of
moles n, universal ideal gas constant R, 'A
TA To.
, and
To
Expert Solution
Step 1 P- V diagram
a) P-v diagram of given process:-
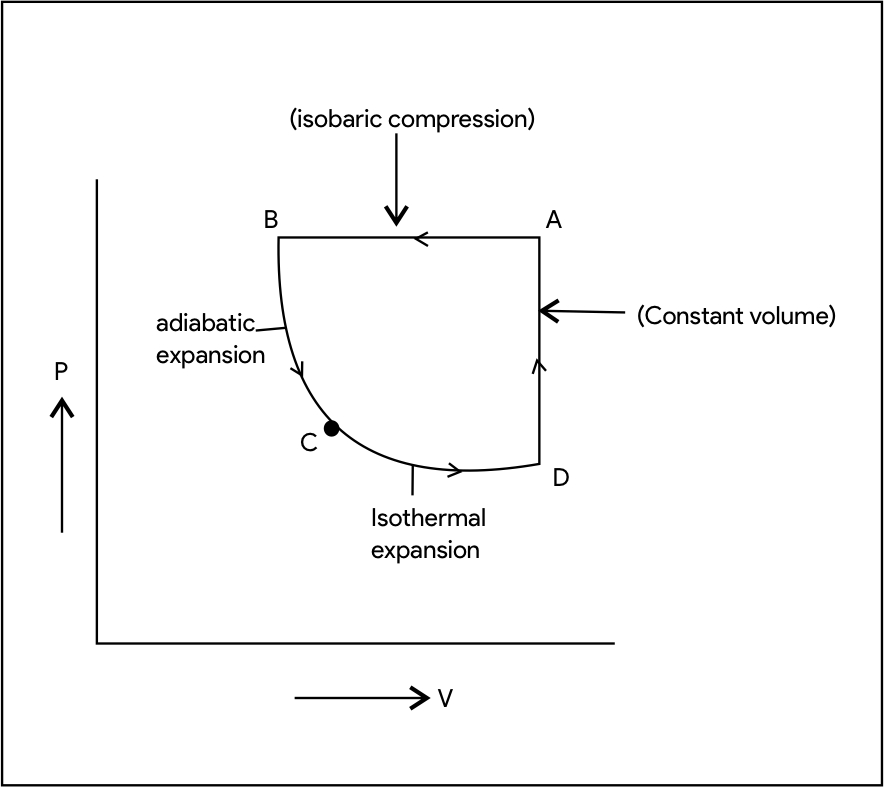
b) For isobaric process AB:-
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY