4. Provide the product for the following reaction. Provide the sterochemistry and regiochemistry where is appropriate. Show the mechanism for each reaction 1) ВНа-THF 2) но , NaOH а) 1) BH3•THF b) 2) HаОг. NaOH 1) BH3•THF 2) H-0г. NaOH c) 1) BH3•THF d) 2) HОг. NaOH 1) ВНа-THF 2) HОг. NaOH 1) BH3•THF 2) HОг. NaOH f)
States of Matter
The substance that constitutes everything in the universe is known as matter. Matter comprises atoms which in turn are composed of electrons, protons, and neutrons. Different atoms combine together to give rise to molecules that act as a foundation for all kinds of substances. There are five states of matter based on their energies of attraction, namely solid, liquid, gases, plasma, and BEC (Bose-Einstein condensates).
Chemical Reactions and Equations
When a chemical species is transformed into another chemical species it is said to have undergone a chemical reaction. It consists of breaking existing bonds and forming new bonds by changing the position of electrons. These reactions are best explained using a chemical equation.
parts a, b, c, d, e, and f

Since you have posted a question with multiple sub-parts, we will solve first three subparts for you. To get remaining sub-part solved please repost the complete question and mention the sub-parts to be solved.
Given reaction:
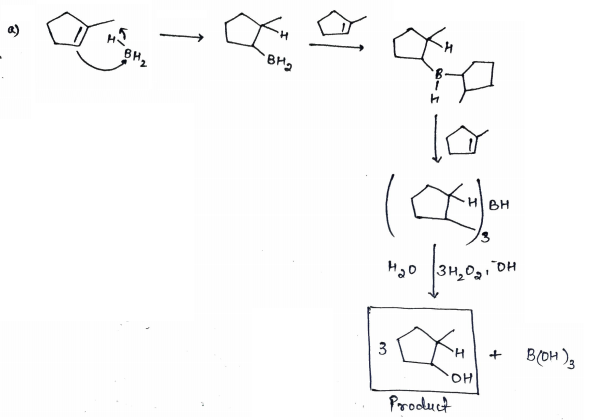
The addition of boron hydride in the presence of tetrahydrofuran and in presence of peroxide in alkaline medium to form alcohol is a hydroboration oxidation reaction. Hydroboration oxidation follows antimarkovnikov rule.
Antimarkovnikov rule states that during addition reaction in alkene, the negative part of the adding molecule will get attached to that carbon which contains more number of hydrogen atoms.
Thus, the mechanism for given reactions is represented as follows:
Step by step
Solved in 7 steps with 5 images









