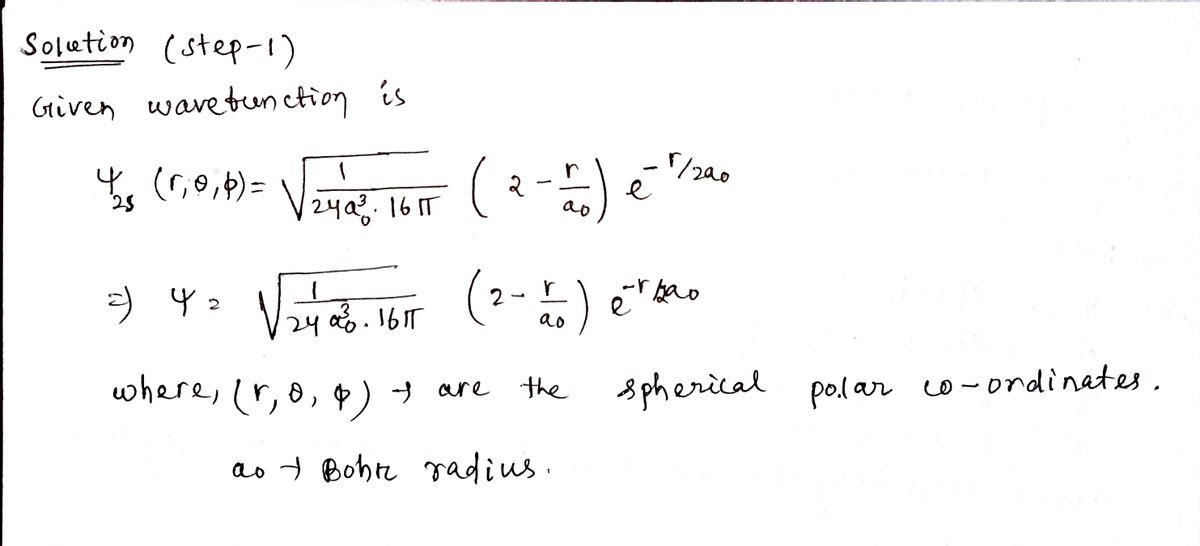
4. Calculate for the normalized wavefunction from # 3.
Q: Consider two charges, where one (+3.60 nC) is at the origin and the other (-13.0 nC) is at the…
A: Given:- The two charges, the first charge is q1=+3.60 nC at the origin The other charge is…
Q: Billy is running away from a Wells Fargo bank at a speed of 13 kilometers per hour (units are km/h).…
A: Given data: Speed (v) = 13 km/h Distance (d) = 1.9 km Required: Time (t)
Q: The answer to (a) is -ve but (b) isn't correct wether +ive or -ive. I'm not understanding (b).
A:
Q: An ant is crawling along a straight wire, which we shall call the z axis, from A to B to C to D…
A: The motion of an ant along x-axis from to to to .The point is origin.The distances are as , and…
Q: The plane carrying the supplies will be cruising at a constant velocity of 250 miles per hour…
A: The given values are,
Q: A loud speaker tuned to a single frequency, fo, is rotating in a circle with constant speed v. For…
A:
Q: 2nrad / sec2 60 rad / sec2 O rad/sec2 n rad/ sec2
A: angular displacement in 1 min is 2π now angular speed is (2π/15)/4 sec = 2π/60 rad/sec
Q: What is the density (in vehicles/km/lane) if I have a 2 lane road, with a total of 60 vehicles, and…
A: Given data: Number of lane = 2 Total number of vehicles = 60 Road length = 500 m = 1/2 km = 0.5 km…
Q: A ball has reached a height of 5.7 meters.The time it took to reach the height was 1 minute 10…
A:
Q: Problem 4 Consider the vectors A and B, with magnitudes given by A = 6 and B = 3. Their directions,…
A: Given, A→=6B→=3θA=45°θB=115° Note : According to our guidelines we'll answer the first 3 subparts of…
Q: I have a couple of follow up questions: 1) Is that 60k^ kgm^2/s, the SI unit for L 2) It wanted…
A: Given data: Mass of the object, m=2 kg Position vector, r→=6 i^+5t j^ m
Q: Four identical charged particles (g = +14.0 µC) are located on the corners of a rectangle as shown…
A: Given : q=14μC L= 64 cm W= 17 cm
Q: Lautaro Martínez, recognized as one of the world’s best soccer players, is playing in the 2022 FIFA…
A:
Q: *3. If an object is projected upward with an initial velocity of 127 ft per sec, its height the…
A: Since you have asked more than three questions, according to our guidelines we would only answer the…
Q: A rocket, initially at rest, is fired vertically upward with an acceleration of 6.0 m/s2. At an…
A: Using kinematic equation, the expression for find fast of the rocket is v2=u2+2asv=u2+2as
Q: 2 3 Which is the correct statement for location 3
A: Amplitude of a wave is the maximum displacement of wave on either side of equilibrium. Its SI units…
Q: In a typical game he runs 8 km. What is this distance in yards?
A: We know , 1km = 1093.613 yards 8kms = 8 × 1093.613 = 8748.906 yards
Q: Question 8 A diver running 1.7 m/s dives out horizontally from the edge of a vertical cliff and 3.2…
A:
Q: this answer barely helps. There isn't any detailed steps or the solution. Can I please get a better…
A:
Q: q Per Sece the object is alt) = -32 feet A ball to thrown Vertically upward from height with an…
A:
Q: A street light is at the top of a 16 ft tall pole. A woman 6 ft tall walks away from the pole with a…
A:
Q: 2. For the 1-D wavefunction: $(x) = Nxe (=²7), where -∞0 ≤x≤00, what is/are the most probable…
A:
Q: 6 of 10 Constants | Periodic Table Part A A spherical cavity of radius 4.50 cm is at the center of a…
A:
Q: You shoot a cannonball off the side of a cliff, and it hits the ground with a horizontal velocity of…
A: Given: The horizontal component of final velocity is 25 m/s. The vertical component of final…
Q: 0 IVE| ΑΣΦ Submit ▾ Part L Request Answer Submit Find the distance the ant moved over the interval…
A: Given AB = 44 cmBC = 20 cmAO = 4 cm
Q: I ended up finding a solution for this question, but I am not understanding the last part of it. How…
A: In the given equation, the variables are replaced respectively with their units. Two physical…
Q: When I was a young adult , I made two trips down the coast of California on a bicycle. If I was…
A: The average speed of the bicycle = 8 miles/hr. Distance to be covered = 2.3 miles.
Q: des constant torque and therefore a constant angular eration a. The flywheel is assumed to be at…
A: The flywheel is initially at rest hence the initial angular velocity will be zero. Rewrite equation…
Q: Which of the following correctly describes A=90090 in scientific notation (with correct sig figs)?…
A: Given values, A = 90090 E# represents 10 raised to the power of #.
Q: Javier Mascherano, recognized as one of the world’s best soccer players, is playing in the 2022 FIFA…
A: Given value--- distance c9vered by soccer player = 8 km. We have to find--- What is this distance…
Q: Three long, parallel conductors each carry a current of I = 1.62 A. The figure below is an end view…
A:
Q: A ring with moment of inertia mr2 and a sphere with moment of inertia 2/5mr2 have the same mass and…
A: Option A. 0.7
Q: 8. The ground reaction force acting on a long jumper is 6700N acting forward and upward at an angle…
A:

The solution for the above problem is given below.
Step by step
Solved in 3 steps with 3 images

- How far up did the stone in the question above made it? O 10 m 90 m O45 m O30 mI’m not sure how I type this answer in.DUE NOW. Please answer it correctly and with complete solution. Please provide the solving in this format: Given, Required, Formula, Solution, and Answer. Please computerize or type the solutions digitally, not written in the paper. Thanks!
- Write the scientific notation (10n) 0012 = 560000 = Write out the number 3 x 104 = 8 x 10-2 = How many centimeters are in 2 kilometers? Write your answer in scientific notation. How long would it take in minutes to travel 5km if you are traveling at a speed of 30 m/s? A ship left shore 3 days ago and has been moving at a constant speed. The cruise ship is now 1440 miles away. What is the average speed in mph? 5a. A tuning fork has a period of 4s. What is the frequency? 5b. If the tuning fork above is struck in air, what is the wavelength? 5c. If the tuning fork above is struck in water, what is the wavelength? The wave below is traveling at 5 m/s. 6a. What are the wave y(x) and oscillator y(t) sinusoid equations for this wave? What is a sound wave? Identify where the compressions and the rarefactions are in both graphs.…Which one I should choose exactly?I'm getting mixed answers for this question. Can I please get someone who is confident in their answer for a) b) and c) ASAP?
- A jogger runs 186 meters westward, then 210 meters eastward, then 156 meters westward, and finally 84 meters eastward. For this motion, what is the distance moved? Tap button at left to enter answer using our built-in number pad. Distance (m) : %D 1 2 What is the magnitude and direction of the displacement? Magnitude (m) = Tap button at left to enter answer using our built-in number pad. Dir'n = (Tap field to change.) --An ant is crawling along a straight wire, which we shall call the z axis, from A to B to C to D (which overlaps A), as shown in the figure below. (Figure 1) O is the origin. Suppose you take measurements and find that AB is 44 cm, BC is 20 cm,and AO is 4 cmA car travels with an average speed of 8.00 meter/second, What is the speed in km/s? What is this speed in mile/hour? Hint: 1km = 1000.0 meters, or 1 m = 0.00100 km; 1 mile = 1.60 km, or 1 km = 0.625 mile; 1hour = 3600.0 seconds